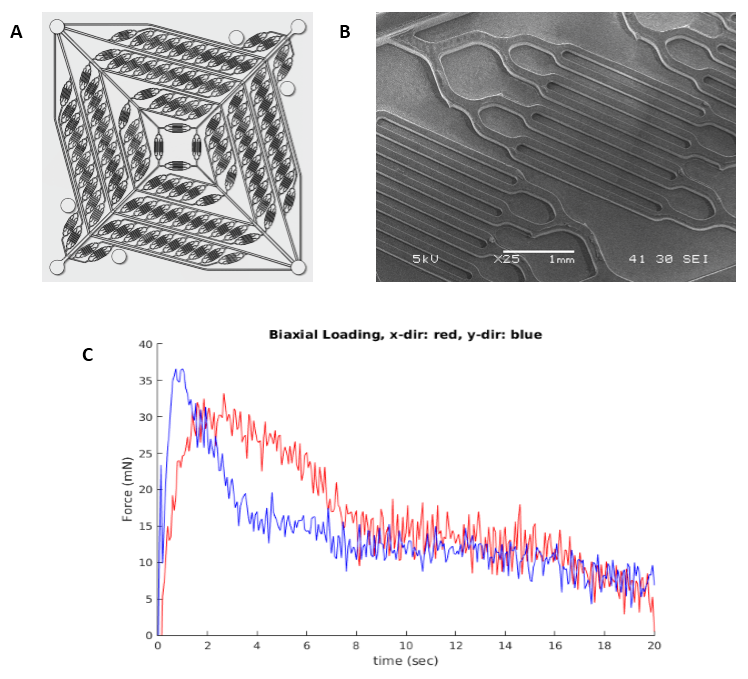
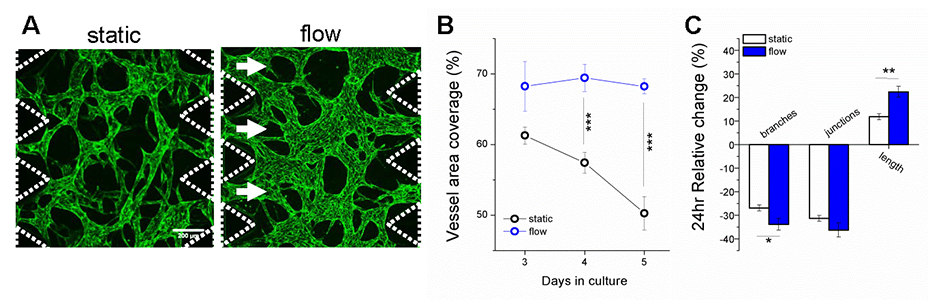
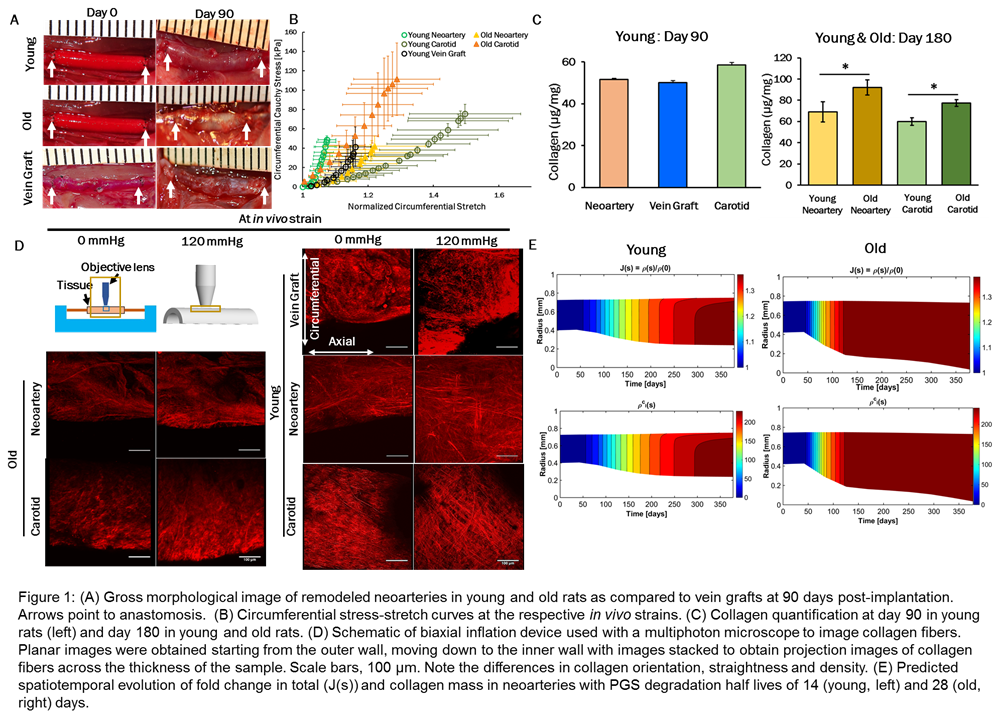
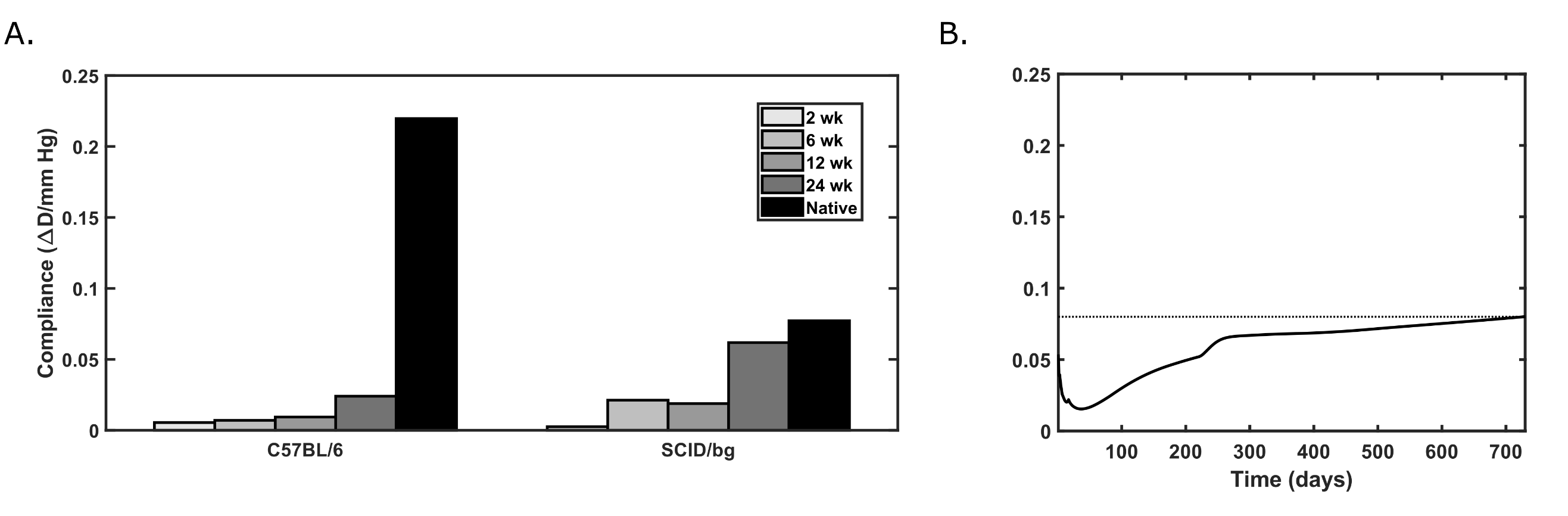
Abstract
Introduction
Globally over 100 million people are affected by heart valve (HV) diseases and 300,000 HV replacements are required annually (Namiri et al., 2015). Mechanical and bioprosthetic HVs are unable to remodel, making replacement surgery the final choice for paediatric patients. Tissue engineered heart valves (TEHVs) promise full integration and remodelling in vivo, eliminating paediatric patients’ need for revision surgeries.
Our TE approach combines autologous cells with a fibrin-collagen-glycosaminoglycan scaffold (Brougham et al., 2017, 2015), conditioned using a bioreactor which simulates the biomechanics of the heart. This encourages directional cell proliferation and extracellular matrix development. The ideal bioreactor parameters to create functional TEHVs remain unknown. Our main goal is to create a pulmonary TEHV suitable for in vivo trials and the first milestone is to design a bioreactor capable of conditioning TEHVs.
Design Requirements
The bioreactor must mimic adult and neonate hearts (30-200 bpm, flow of 25-100 ml/beat and pressure drop of 13-25 mmHg throughout the HV). It must be fabricated from non-cytotoxic materials, sterilisable using ethylene oxide and/or autoclaving, allow observation of leaflets, while promoting gas and media exchange and remaining sterile.
Design Concept
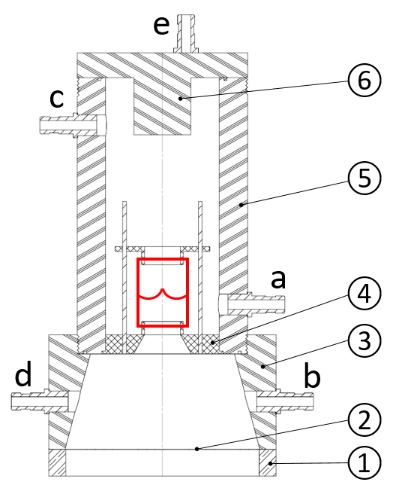
In this design (Figure 1), the HV is fixed aseptically inside the pulmonary chamber of the bioreactor. Media is pumped through the HV by displacing a silicone membrane, using a magnetic actuator, secured in a stainless steel base. Silicone tubing will allow displaced media to circulate between polypropylene connectors (a-b). A peristaltic pump will pump media between connectors (c-d). The bioreactor itself will be fabricated from PMMA allowing clear observation of the HV and leaflets. A compliance chamber and a syringe filter will be attached to connector (e) to simulate arterial expansion and promote gas exchange.
Figure 1. Bioreactor design showing HV in red. Contains a base (1), silicone membrane (2), ventricular chamber (3), HV support (4), pulmonary chamber (5), lid (6) and connectors (a-e).
Discussion
Our bioreactor is loosely based on the conceptual design proposed by Hoerstrup et al. (2000), and the bioreactor designed by Moreira et al. (2014). Unlike those designs, the ventricular chamber and HV support act as nozzle directing the flow towards the HV. Operating parameters across the HV will be validated using pressure transducers and flow sensors. Cytotoxicity and sterility tests will validate materials selected. In time, this bioreactor will be employed to condition TEHVs using a variety of conditioning regimes.
References
Brougham et al., Advanced Healthcare Materials 6: 21 2017.
Brougham et al., Acta Biomaterialia 26: 205-214, 2015.
Hoerstrup et al., Tissue Engineering 6: 75-79, 2000.
Moreira et al., Tissue Engineering 20: 741-748, 2014.
Namiri et al., Journal of Tissue Engineering and Regenerative Medicine 11(5): 1675-1683, 2017.
Acknowledgments: DIT Dean of Graduate Research School Award 2017-2021