Abstract
Stroke is a condition that affects over 15 million people worldwide every year. There are two types of stroke: ischaemic, which is the more common and occurs when blood supply to the brain is blocked, and haemorrhagic, where blood vessels in the brain burst, causing bleeding. After the occurrence of an ischaemic stroke, one typically observes three regions being formed, namely the ischaemic core, penumbra and the unaffected region. While cells in the ischaemic core die immediately due to lack of blood supply, there remains a chance to salvage those in the penumbra, as these cells are still supplied temporarily by peripheral vessels.
Where to place the borders demarcating these three regions is an issue that many researchers have investigated, yet with limited success. To this end, our goal is to create a mathematical model that uses cell calcium ion concentration as a biomarker to predict cell survival rates in these three regions of the brain, given some decrease in blood flow, a clinically measureable input. This would allow our model to be used as a diagnostic tool for clinicians to determine which areas to apply reperfusion.
We find that our model is able to portray certain spatial inhomogeneities known as Turing patterns, which arise from diffusion-reaction equations satisfying certain conditions. Specifically, the patterns occur when the system is linearly stable in the absence of diffusion but unstable in its presence. A further condition arises from the boundary conditions: only certain modes are admissible for a specific set of domain and boundary conditions. We can find, subsequently, a set of parameter values satisfying these criteria.
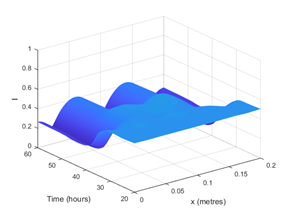
Hence, with only one set of differential equations expressing calcium concentrations as the sole biomarker, we are able to show that the resulting simulations can form spatial inhomogeneities. An example of this is shown in Figure 1, where we plot the injury (I) profile over space and time: this variable is our measure of cell damage to different regions of the brain. The results show that a region of instability is found under these particular conditions.
This is a feature that, to our knowledge, has not been investigated in other models on ischaemic stroke. The presence of Turing patterns allows us potentially to model the ischaemic core, penumbra and unaffected regions; however, the spatial inhomogeneities observed in our model simulations do not yet correspond to these regions due to the effects of higher-order modes. We, therefore, intend to further investigate this behaviour and to expand the model in order to relate it to clinical data in the future.
 [1] R. Ross, Nature, vol. 362(1993), pp. 801
[1] R. Ross, Nature, vol. 362(1993), pp. 801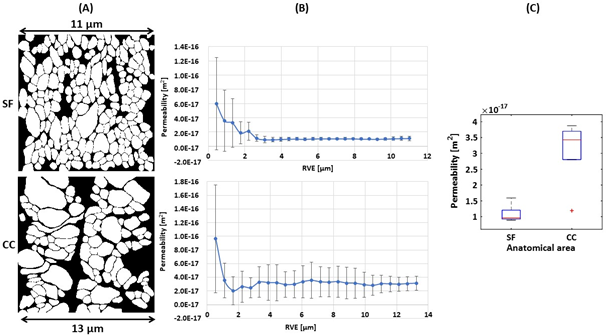
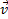 /dt where
/dt where