Abstract
Background
The mammalian meninges are composed of three thin and heterogeneous layers of highly organized tissue with a variety of functions, from regulating the development of brain cortex to isolating the central nervous system [1]. Despite their putative role as structural dampers helping to mechanically shield the brain, their mechanical properties are still poorly investigated [2]. Here, we characterized the local mechanical heterogeneity of rat leptomeninges at the microscale via atomic force microscopy (AFM) indentation experiments to understand how microstructural variations at the tissue level can differentially affect load propagation.
Methods
Rat pia-arachnoid complex (PAC) were isolated from rat brains immediately after death. The thin layers of tissue were peeled-off under a dissecting microscope and left to float in PBS. These were then adhered to tissue-treated glass slides. AFM indentation curves were performed on different positions of leptomeninges immersed in PBS using tipless cantilevers with spherical beads (R=30 μm). A hyperelastic material model (Fung) was used to fit the curves [3].
Results
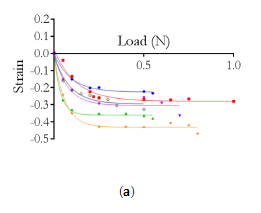
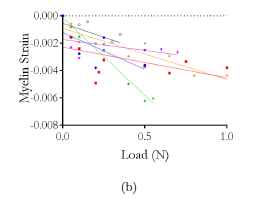
Indentation curves were acquired for increasing indenter speeds on different positions of the PAC; increasing hysteresis (indicating viscoelastic material behavior) was observed for non-vascularized portions of the tissue, whereas indentations on capillaries yielded overlapping force-displacement cycles (Fig.1a). The approach curves were analyzed via a Fung hyperelastic model and indicated a 20-fold increase in the initial Young’s modulus E0 of vascularized tissue (8.74 ± 0.73 kPa) as compared with non-vascularized PAC (0.38 ± 0.10 kPa); even within the latter, local heterogeneity in collagen content caused varying stiffness values (Fig.1c).
Figure 1: (a) Sketch of PAC isolation and AFM measurements. (b) AFM force-distance curves recorded at different loading rates for a single position on non-vascularized vs. vascularized PAC tissue. (c) Indentation portion of AFM curves for three different positions on non-vascularized PAC tissue and corresponding Fung fits.
Discussion
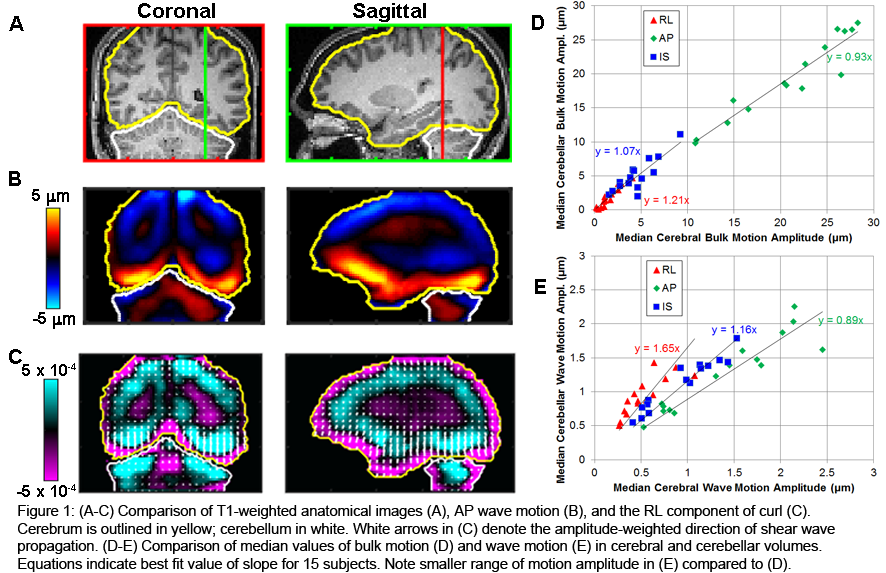
It is known that leptomeninges display a high regional variability both in arachnoid trabeculae and subarachnoid vasculature distribution [4]. Our data highlight strong local variations in the mechanical response of this tissue, possibly relating to the differences in local vulnerability to TBI reported by [5]. In the future, we will couple such mechanical micro-indentations with confocal microscopy Z-stacks, to directly observe how external loads propagate and deform the subarachnoid space trabeculae.
Acknowledgements
NSF award (CMMI 1728186). We thank Dr. Costa (Icahn School of Medicine, Mount Sinai, NY) for AFM access.
References
[1] Decimo, I. et al., (2012), Am J Stem Cell, 1(2):92-105
[2] Jin, X. et al., (2011), J Biomech, 44:467-474
[3] Lin, D.C., et al., (2009), Biomech Model Mechanobiol, 8(5):345-358
[4] Scott, G.G. et al., (2015), Trans Med Imag, 34(7):1452-1459
[5] Scott, G.G. et al., (2016), Biomech Model Mechanobiol, 15:1101-1119
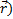 and the estimated impact versor (
and the estimated impact versor ( 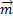 ):
):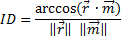
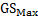 ):
): 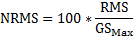
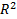 .
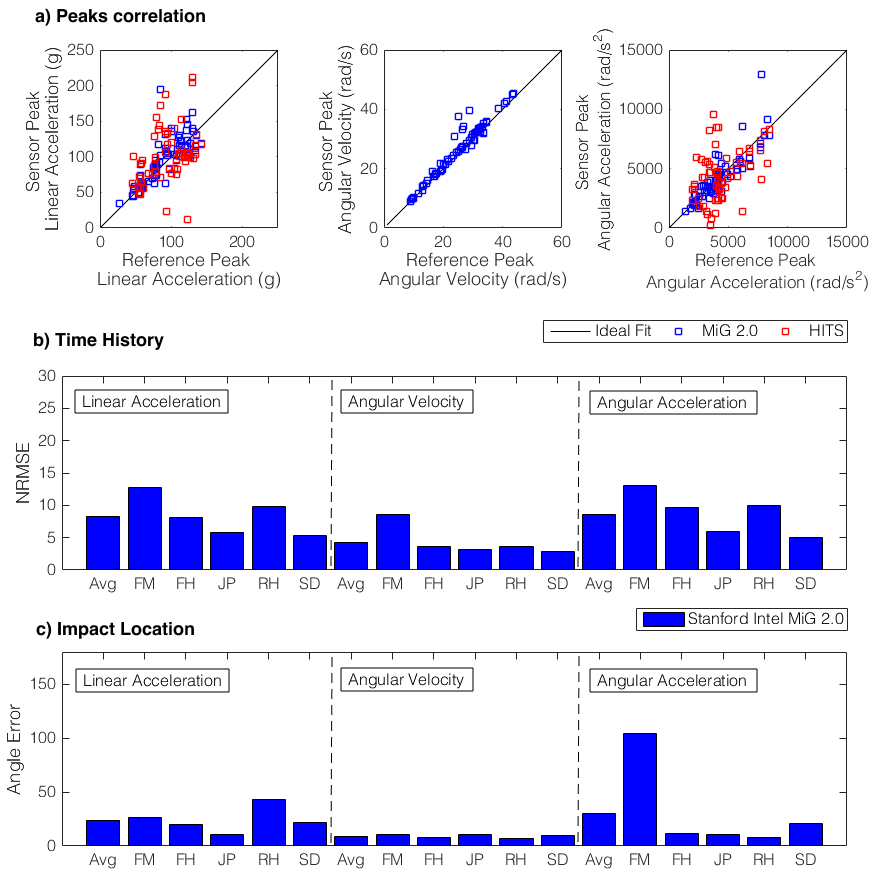
.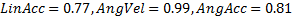 ), followed by HITs (
), followed by HITs (  ) (Fig 1). MiG 2.0 also showed low average NRMS error (
) (Fig 1). MiG 2.0 also showed low average NRMS error ( 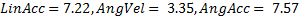 ) and impact direction error (
) and impact direction error ( 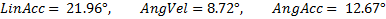 ).
).