Abstract
Introduction: Anterior cruciate ligament (ACL) injuries are increasingly prevalent in children and adolescents. Known changes in ACL orientation in humans [1] suggest a potential need for age-specific clinical treatments. Yet, much is unknown regarding ACL function during growth at the joint and tissue levels. Our recent data has shown age-specific force distribution within the anteromedial (AM) and posterolateral (PL) ACL bundles [2]. The current objective was to determine if observed age-dependent joint-level changes in function correspond to tissue-level changes in ACL size and function. We hypothesized that the size and stiffness of the AM and PL bundles would undergo bundle-specific changes during growth.
Methods: Hind limbs were collected from 36 female Yorkshire-cross pigs at ages ranging from birth through skeletal maturity (6 groups, n=6/age). Each limb was imaged in a high-field MRI scanner and scans were processed using image segmentation software [3]. Cross sectional area (CSA) data were calculated for the AM and PL bundles. Functional testing was performed with a 6 degree-of-freedom force sensing robotic system to apply defined loads and moments simulating clinical exams to the joints and determine joint kinematics and tissue contributions to load resistance [4]. Statistical analysis included one-way or two-way ANOVA testing and Tukey’s post-hoc analysis.
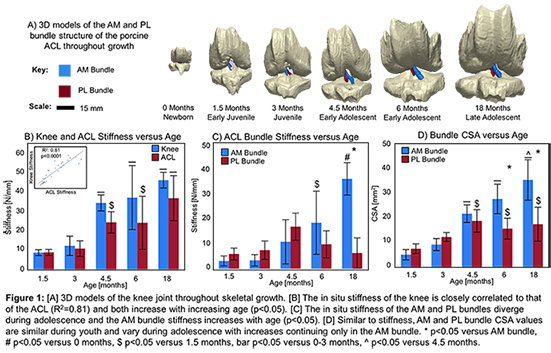
Results: Mean knee stiffness increased by 483% from 1.5 months to 18 months under applied loading (Fig. 1B). ACL stiffness increased by 468% over the same age range. Knee and ACL stiffness were closely correlated across all age groups (R2:0.81-0.93). AM bundle stiffness also increased by 383% by 18 months, whereas the PL bundle stiffness reached a plateau at 4.5 months of age (Fig. 1C). Structurally, the CSA of the AM and PL bundles were similar in young groups (p>0.05), while the CSA of the AM bundle was significantly greater in mid- and late-adolescent groups (p<0.05) (Fig. 1D).
Discussion: Although the porcine ACL plays a primary role in stabilizing the knee under anterior drawer throughout post-natal growth, the stiffness and CSA of its AM and PL bundles undergo bundle-specific changes during growth. These changes explain age-dependent differences in the bundle contribution to resisting anterior drawer loads and valgus torques as observed previously [2]. These changes occur alongside previously noted changes in ACL orientation [3]. Ongoing work is focusing on additional intrinsic bundle mechanical and biochemical properties. Overall, these data highlight underappreciated, bundle-specific changes to the ACL during post-natal growth and should be considered when establishing clinical interventions for ACL injuries in young patients.
Acknowledgements: Funding provided by NSF GRFP (DSE-1252376) and NIH (R03 AR068112).
References: [1] HK Kim+, J Pediatr Orthop, 2012, [2] SG Cone+, SB3C 2017, [3] SG Cone+, J Orthop Res, 2017, [4] MB Fisher+, J Orthop Res, 2010.