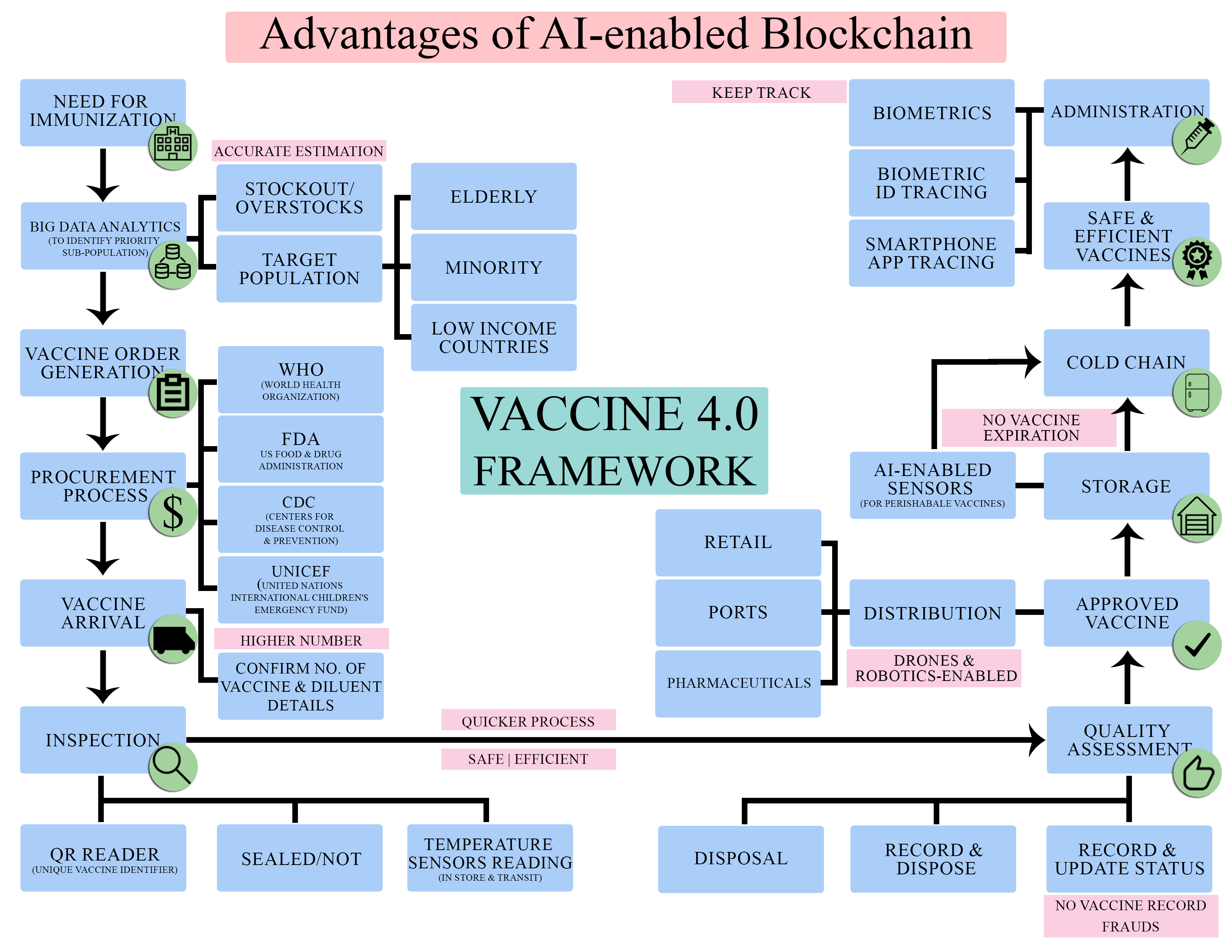
Abstract
We have modeled the kinetics of SARS-Cov-2 specific IgG, based on a single patient case (an Italian 57-year-old male, living in Pesaro (a province of Marche region being one of the main outbreaks of the epidemic in Italy), manifested the onset of symptoms (non-respiratory, but with diarrhea, asthenia, articular and muscle pain, anosmia, and ageusia), limited to the descending phase (during the ascending phase, the immunometric assays were not available yet).
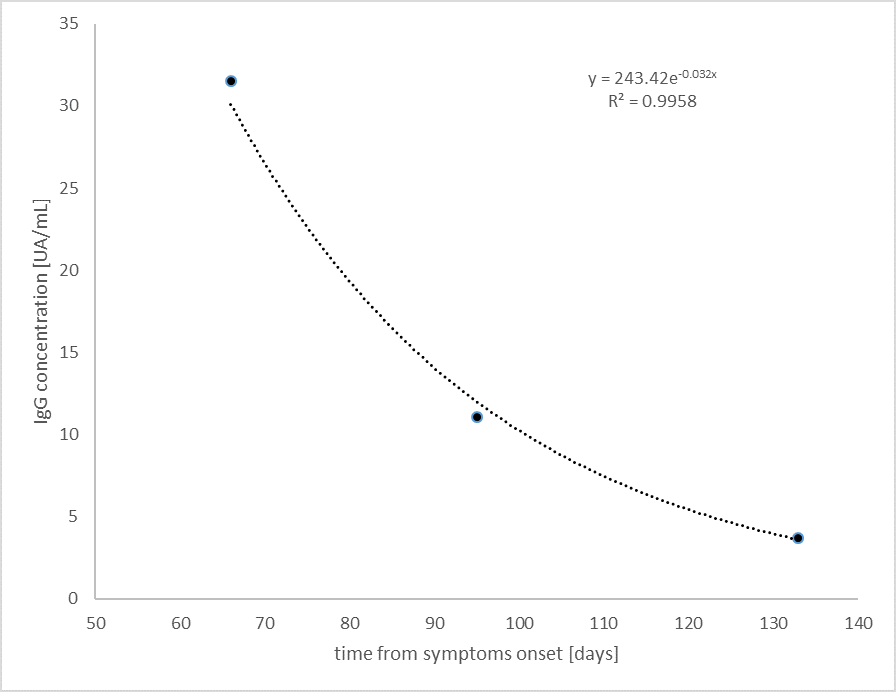
The blood samples were taken in the days 66, 95, and 133 from the beginning of the symptoms, and they were analyzed by CLIA chemiluminescence method, Megalumi® SNIBE.
Typical equations of first-order kinetics were used to describe the trend of the IgG concentration so that a negative exponential function was obtained:
C = a e-kt (1)
With some easy mathematical passages, from (1) we obtain
ln(C) = -kt + ln a (2)
The graph of the models is shown in the figure below. The results show an excellent determination coefficient (R2 = 0.996). once we have the equation parameters, it is possible to calculate both the half-life of IgG (18 days) and the time of their negativization (170 days).
This result does not necessarily imply a loss of immunity at the expected time of negativization. In fact, as some authors have recently highlighted, a cell-mediated immunity could exist. However, our data may suggest the impossibility of identifying the subjects coming into contact with the virus for more than the time of negativization (in our case about six months since the onset of symptoms).
Despite the expected inter-subject variability, the perfect adaptation of the negative exponential model seems to be very promising and it suggests that it could fit equally well for other cases, with different parameters.