Abstract
Polymers of Intrinsic Microporosity (PIMs) combine the desirable processability of polymers with microporosity generated from the inefficient packing of their rigid and contorted macromolecular structures. They are attracting attention for a number of applications but especially as membrane materials for gas separations, pervaporation, organic solvent nanofiltration and as separators in batteries. The ultimate aim of our research is the development of PIMs with enhanced permeability and selectivity for these applications. In particular, we focus on PIMs with exceptionally rigid polymeric structures (e.g. PIM-Trip-TB) and those with macromolecular chains of 2-dimensional shape (e.g. PIM-TMN-Trip), which demonstrate permeabilities similar to those of the ultrapermeable polymer PTMSP but with much higher selectivities.1 Recent work with 2D benzotriptycene based PIMs has resulted in the proposed revision of the CO2/CH4 and CO2/N2 Robeson upper bounds.2 We will describe how further structural and chemical modifications to PIMs results in attractive enhancement of properties for gas selectivity and performance and as membrane separators in redox flow batteries with high cation flux and low cross-over of redox active species.3
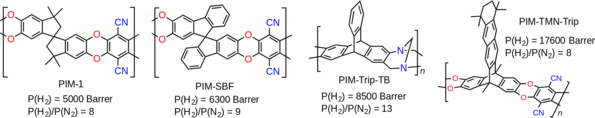
Fig.1. The evolution of hydrogen permeability using PIMs.
[1] I. Rose et al., Nature Materials, 2017, 16, 932.
[2] B. Comesaña-Gándara, et al., Energy & Environmental Science, 2019, 12, 2733.
[3] R. Tan et al., Nature Materials, 2019, doi.org/10.1038/s41563-019-0536-8