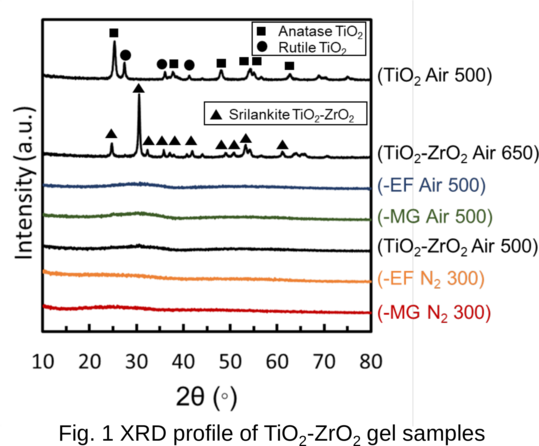
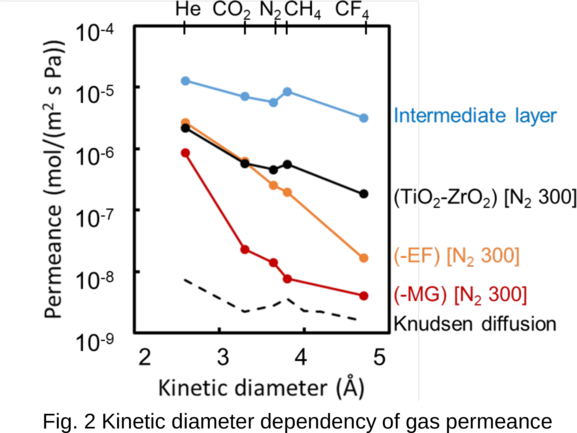
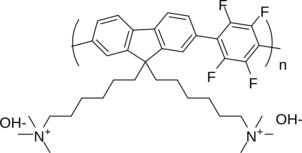
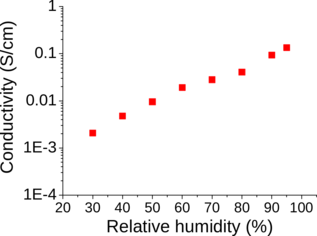
A new class of metal-organic frameworks (MOFs), known as hybrid ultramicroporous materials (HUMs) with high CO2 adsorption capacities [1], have been recently introduced to solve the problems related to water stability, using strong interactions based on tight fitting. They have pore apertures, which are often less than 0.5 nm [2-4].
From the existing HUMs, TIFSIX-3-Ni (for simplification; TIFSIX from now on) is one of the most promising candidate that has one of the highest CO2 capacities (40 cm³ g-1 at 2 mbar CO2 pressure) [5,6].
Powder form of TIFSIX has been investigated for gas separation and purification [7] whilst TIFSIX film synthesis procedure has not studied yet. The transfer of powder synthesis to membrane synthesis is often challenging. Additional properties such as film thickness and roughness have to be considered in order to create high quality films. Furthermore, the formation of films and membranes of MOFs is highly dependent on the specific structure to be studied, the nature of the substrate and the interaction between the two [8-10].
In this study, the thin-film synthesis of TIFSIX on glass substrates are reported by employing several methods including dip-coating, seeding/secondary growth, vapour-assisted conversion, rapid thermal deposition and in-situ coating. Using the in-situ approach with DMF as solvent, we were able to grow homogeneous TIFSIX films at relatively low temperatures and reduced reaction times.
We present the thin-film formation of TIFSIX by a straightforward in-situ synthesis without a need of surface modification on a glass support via SEM, XRD and XPS. We also reported a fabrication methodology based on seeding and secondary growth for TIFSIX films on ceramic tubular substrates and CO2 and CH4 permeabilities.
References
1 O. Cheung and N. Hedin, RSC Adv., 2014, 4, 14480–14494.
2 L.-Y. Lin and H. Bai, Micro. Meso. Mat., 2010, 136, 25–32.
3 M. M. Maroto-Valer, Z. Tang and Y. Zhang, Fuel Process. Technol., 2005, 86, 1487–1502.
4 S. Choi, J. H. Drese and C. W. Jones, ChemSusChem, 2009, 2, 796–854.
5 M. Kandiah, M. H. Nilsen, S. Usseglio, S. Jakobsen, U. Olsbye, M. Tilset, C. Larabi, E. A. Quadrelli, F. Bonino and K. P. Lillerud, Chem. Mater., 2010, 22, 6632–6640.
6 X. Y. Chen, V.-T. Hoang, D. Rodrigue and S. Kaliaguine, RSC Adv., 2013, 3, 24266–24279.
7 H. S. Scott, A. Bajpai, K.-J. Chen, T. Pham, B. Space, J. J. Perry and M. J. Zaworotko, Chem. Commun., 2015, 51, 14832–14835.
8 A. Cadiau, K. Adil, P. M. Bhatt, Y. Belmabkhout and M. Eddaoudi, Science, 2016, 353, 137–140.
9 L. T. Ngyen, K. K. LE and N. T. PHAN, Chinese J. Catal., 2012, 33, 688–696.
10 A. R. Millward and O. M. Yaghi, J. Am. Chem. Soc., 2005, 127, 17998–17999.
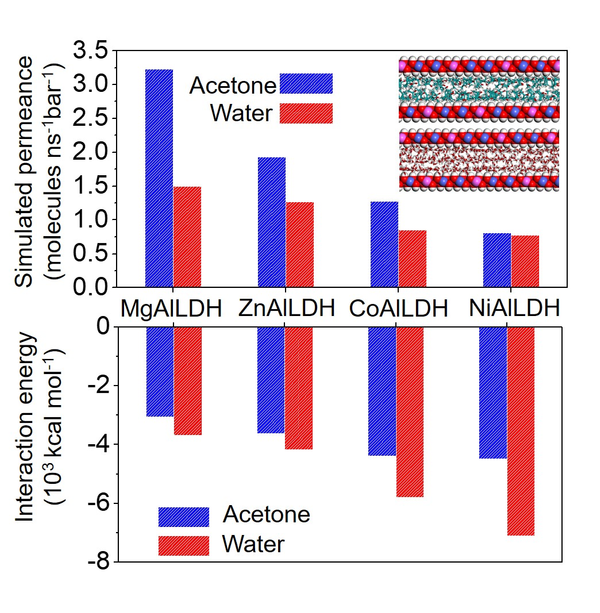
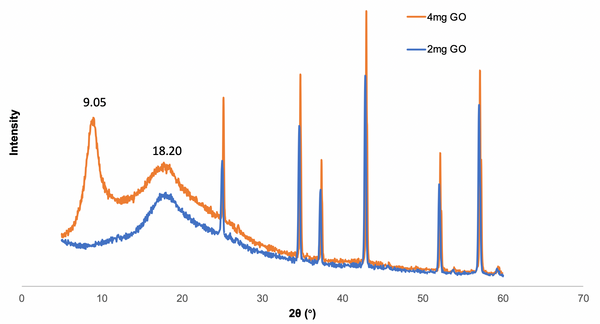
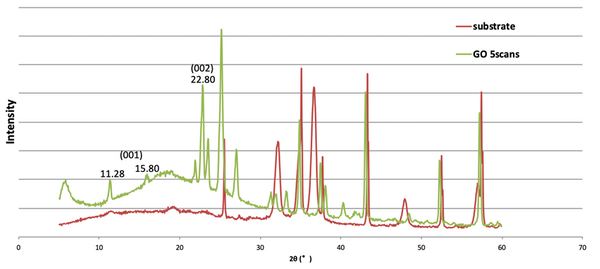
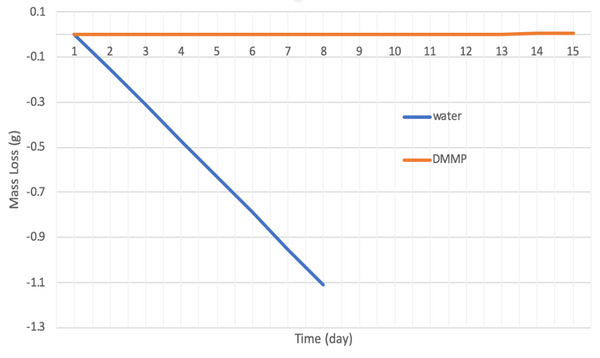
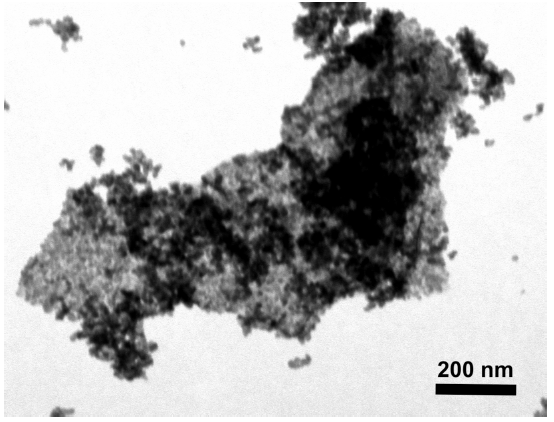
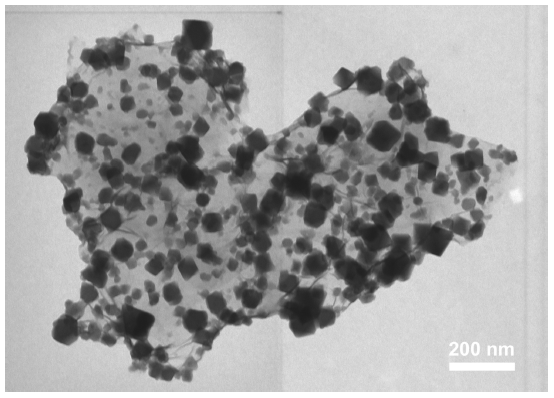
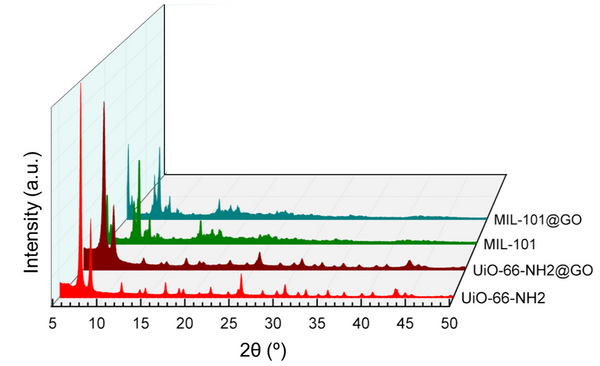
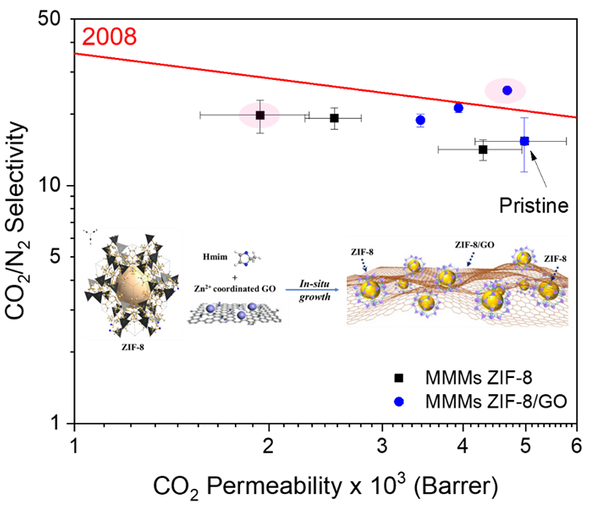
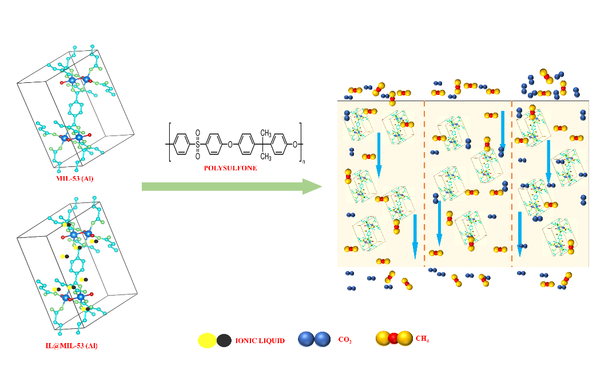
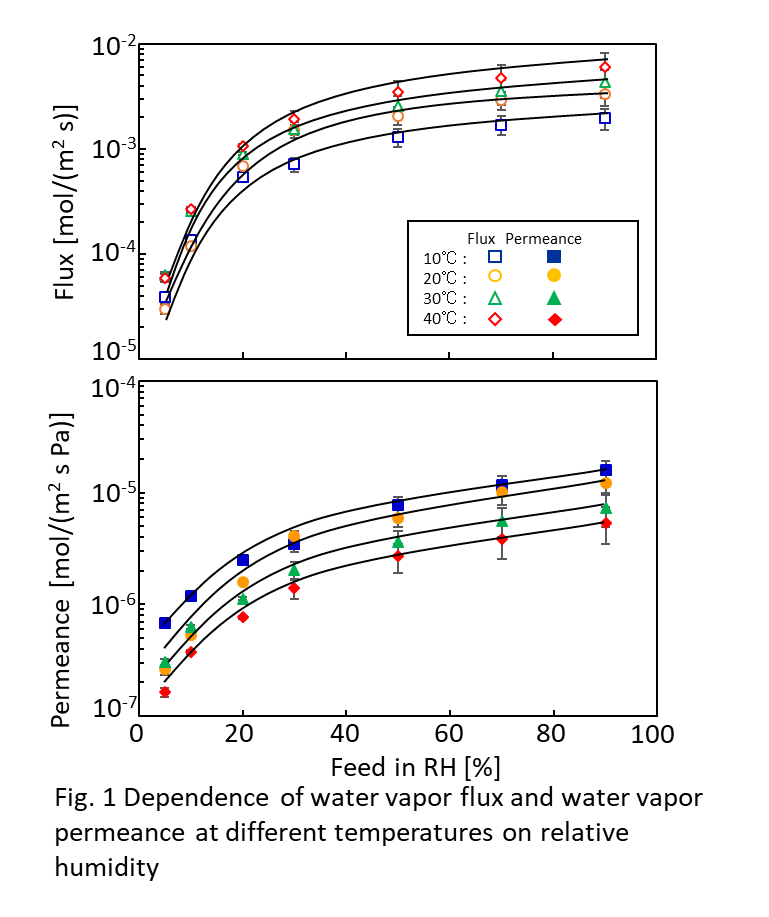
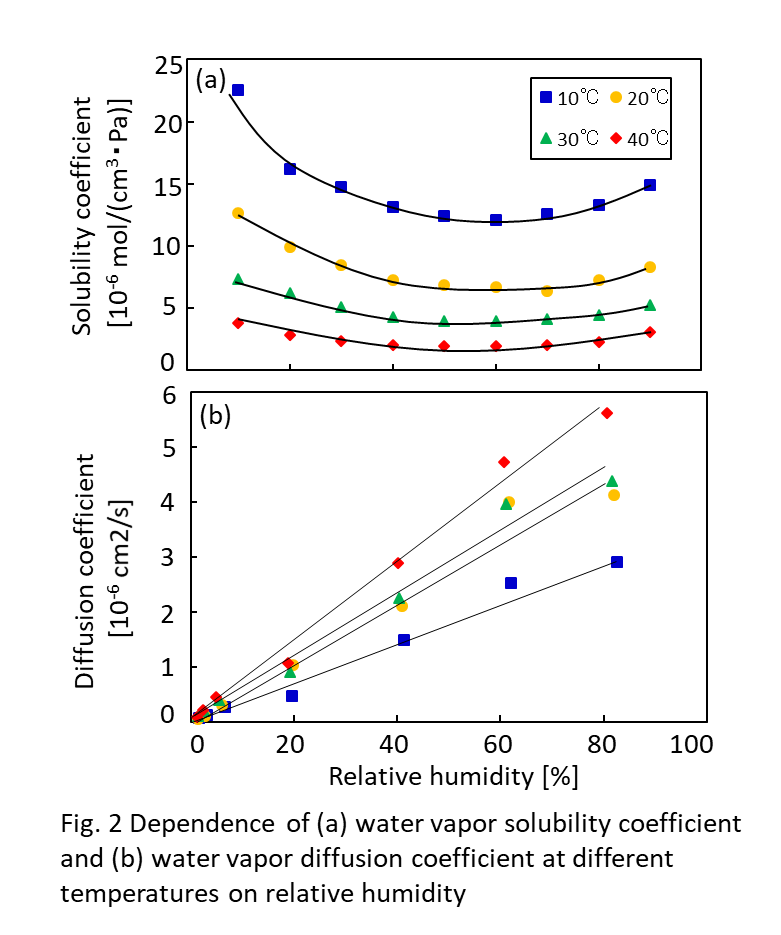
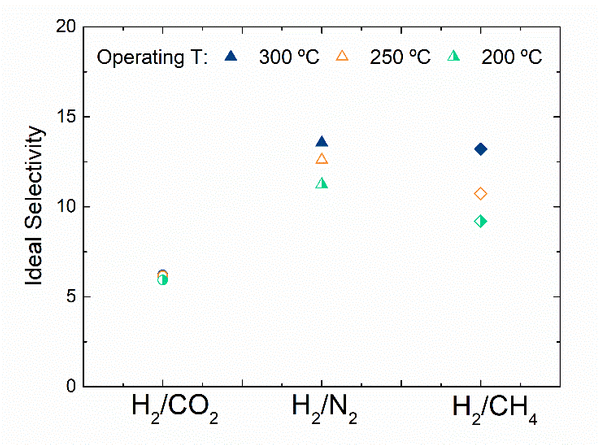
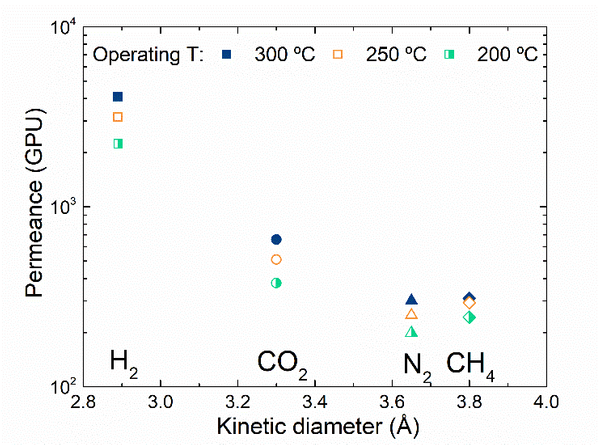
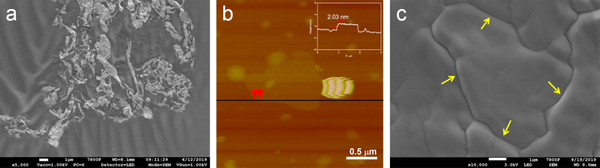
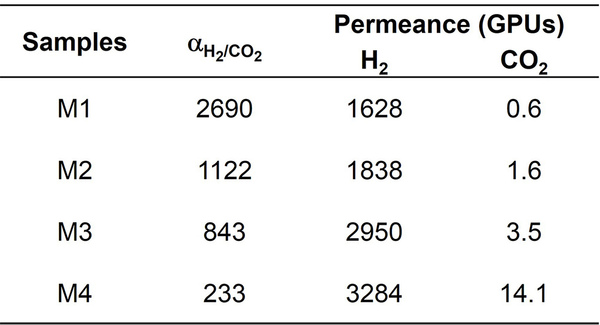
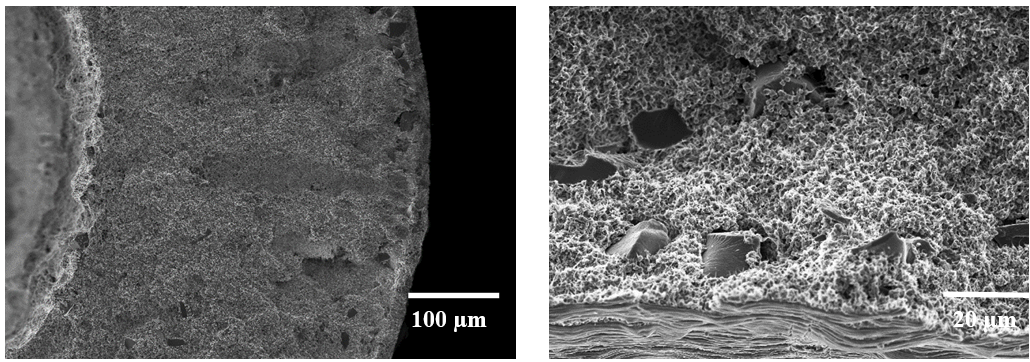
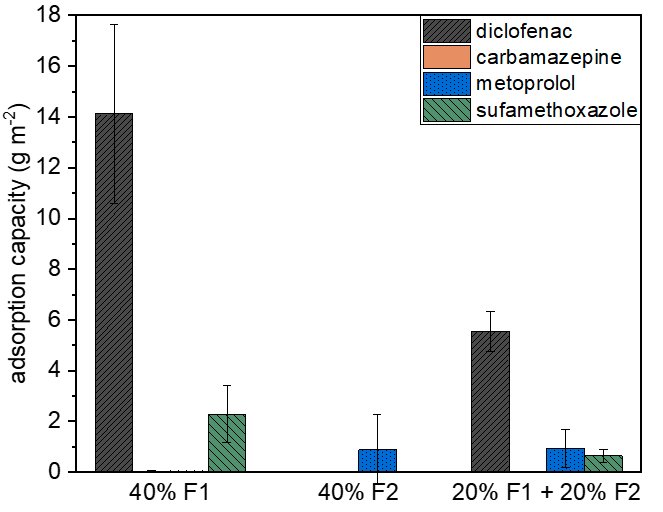
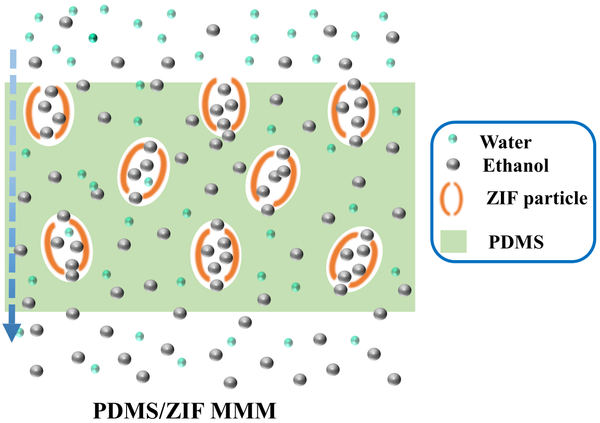
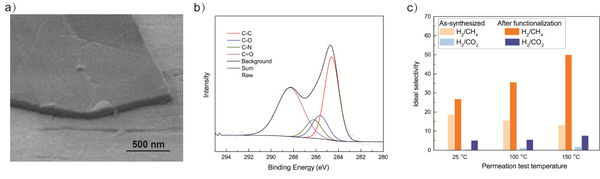 Fig. 1. a) Cross-section SEM image of a 100nm-thick CMS film. b) XPS spectra from the oxygen functionalized CMS film. c) Gas selectivity of CMS membranes before and after the ozone treatment.
Fig. 1. a) Cross-section SEM image of a 100nm-thick CMS film. b) XPS spectra from the oxygen functionalized CMS film. c) Gas selectivity of CMS membranes before and after the ozone treatment.