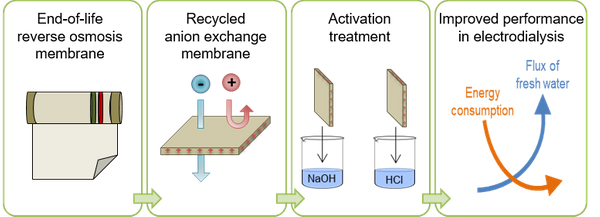
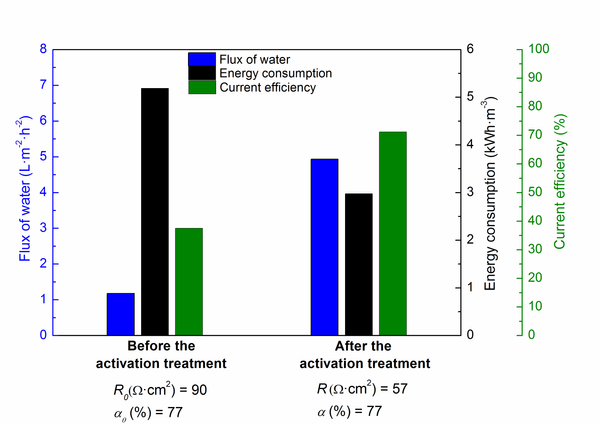
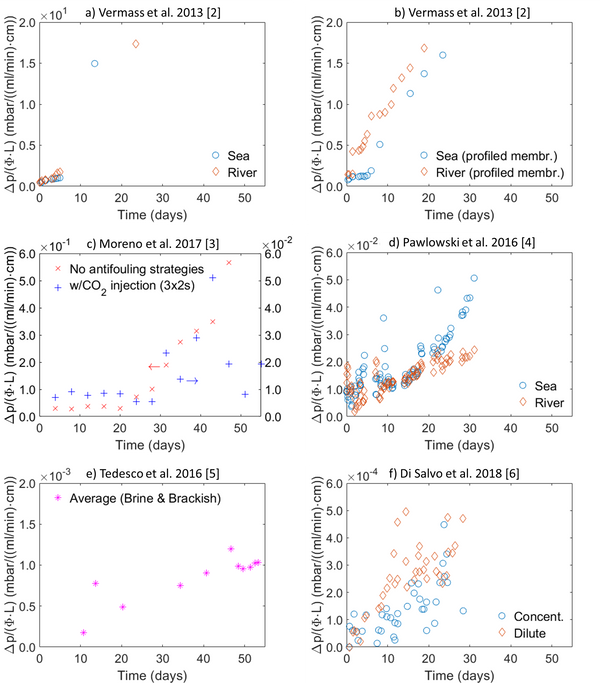
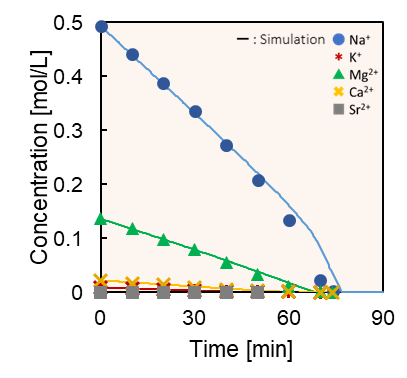
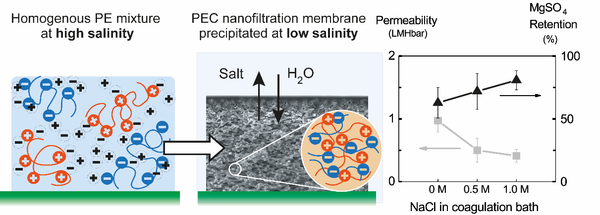
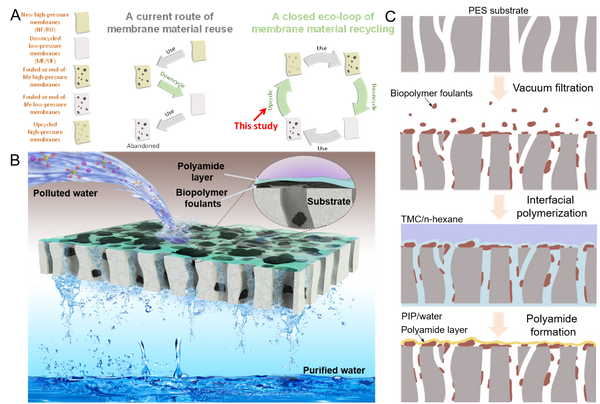
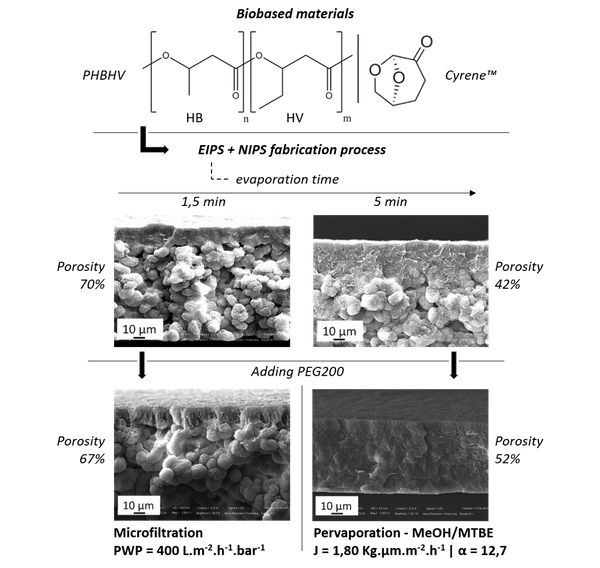
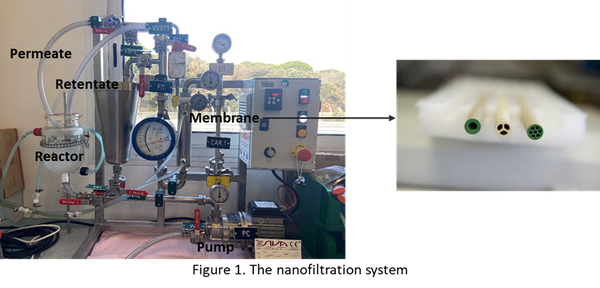
Abstract
Membrane technologies in the last few years have significantly grown in diverse market segments. However specific, attractive applications such as purification of bio-fluids for extracellular vesicles (EVs) isolation have not systematically been explored yet. EVs have recently captured a lot of interest due to their diagnostic significance as biomarkers for diseases, or their potential as drug delivery vehicles and therapeutic agents. The major challenges faced by current membranes are non-specific binding of matrix compounds such as proteins and hence fouling, or the affinity of negatively charged EVs towards the membranes, both result in a very low recovery. The aim of this work is to develop functionalized polymeric membranes, scalable using an industrial approach in an attempt to enhance the recovery of EVs from biofluids.
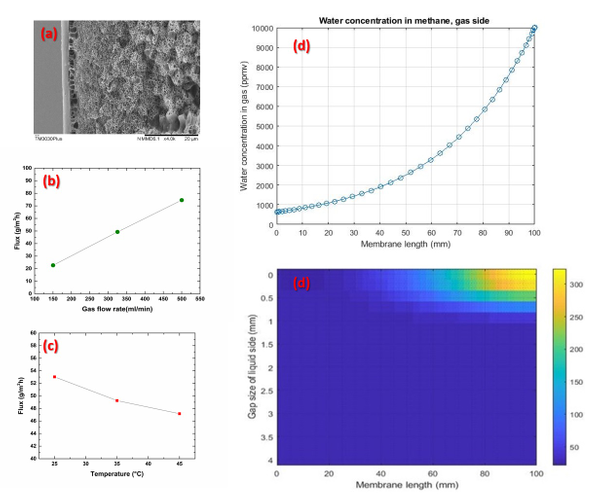
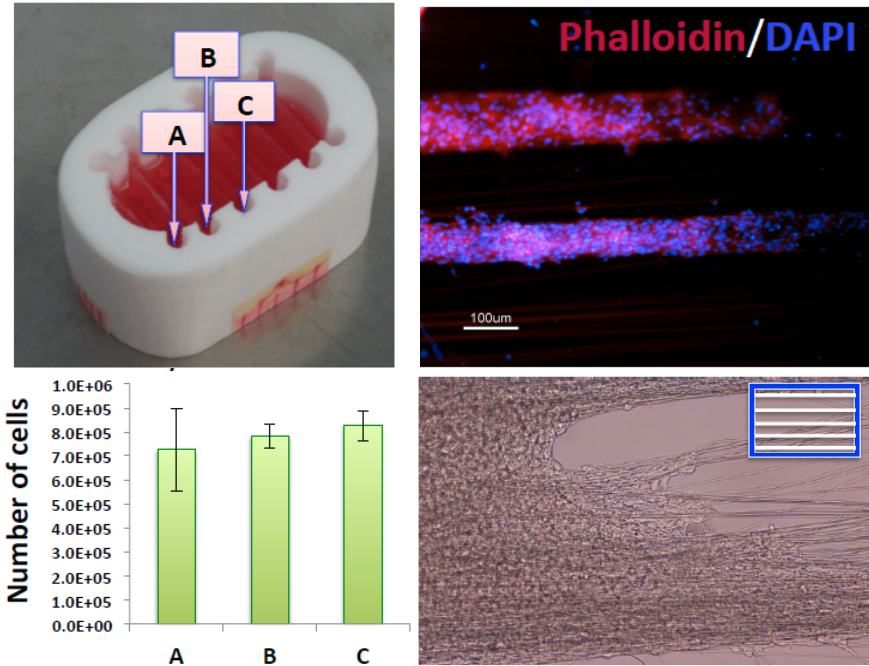
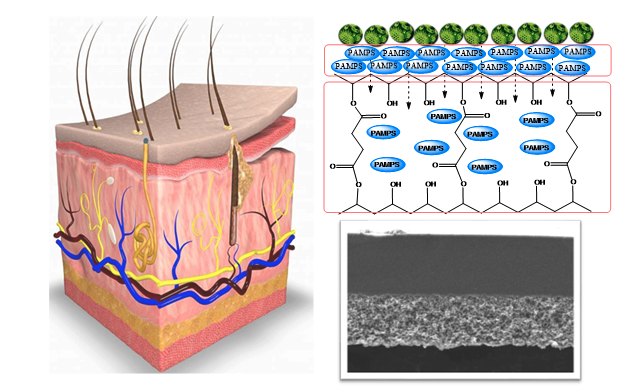
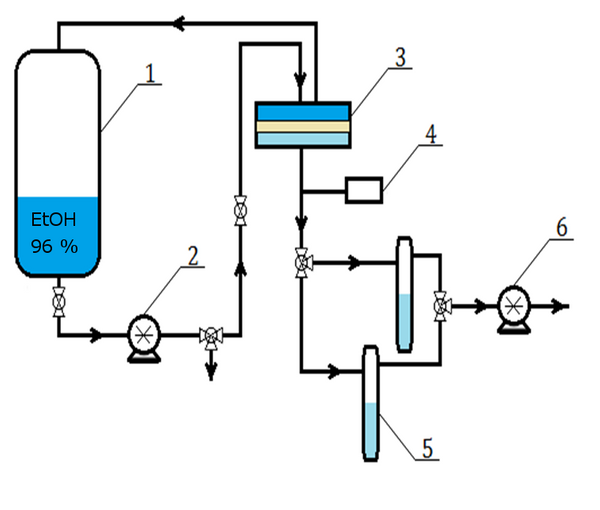
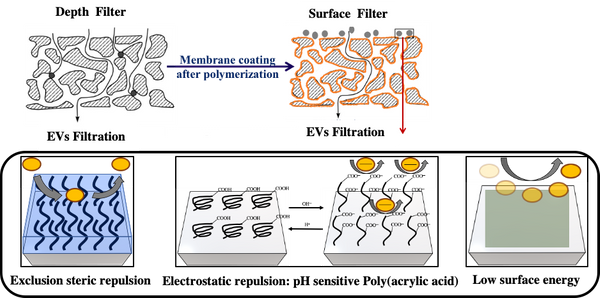
For this purpose, hydrophobic poly(vinylidene difluoride) (PVDF) depth filter membrane is modified into a surface filter by cross-linking it with different building blocks, hydroxyethylmethacrylate and acrylic acid or polyvinylpyrrolidone, using free radical grafting-cross-linking polymerization to obtain 3 prototypes with different surface chemistry (Figure 1). These membranes were evaluated to explore their potential for EVs, with a designed filtration tool which included fluorescent particles of various sizes towards understanding the blocking mechanism to identify the factors affecting efficiency of filtration.
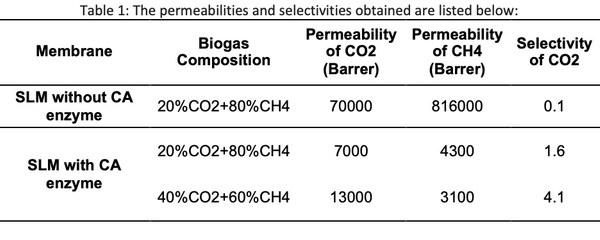
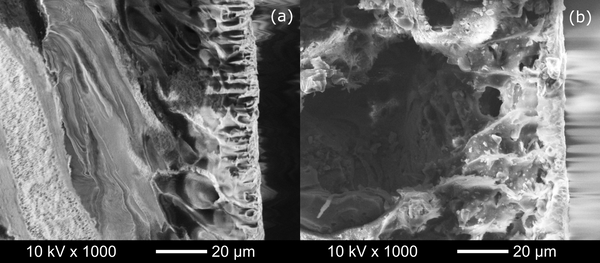
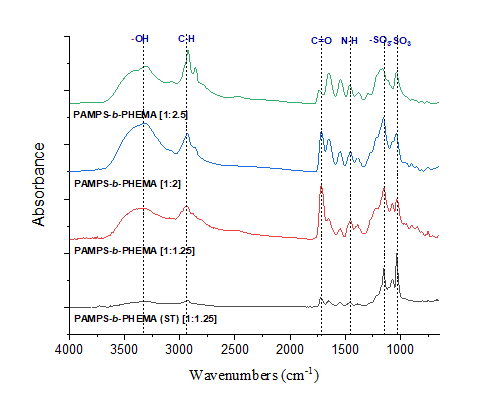
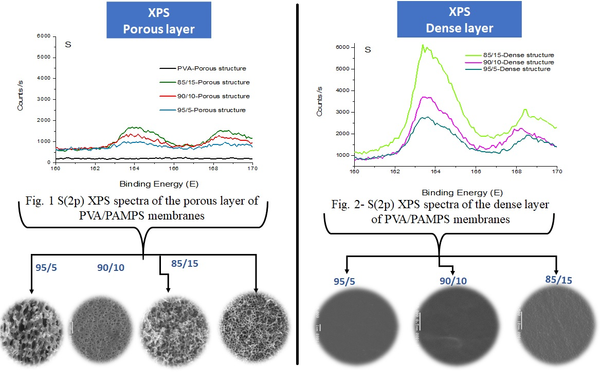
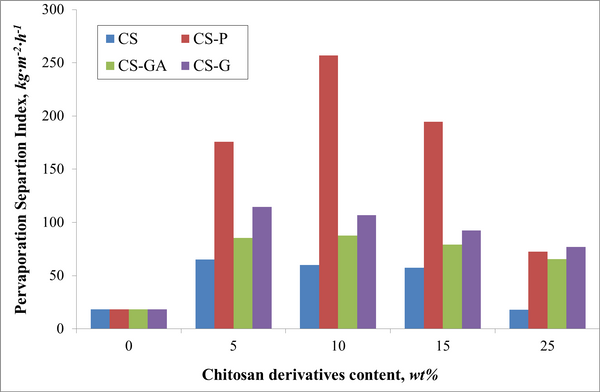
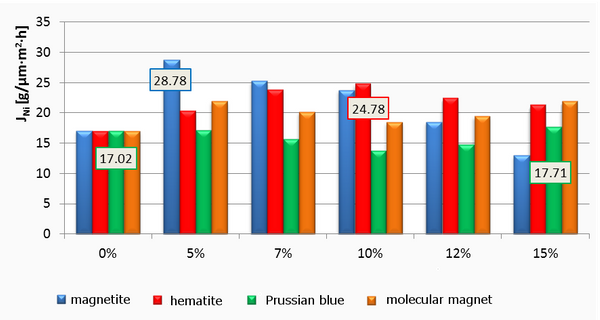
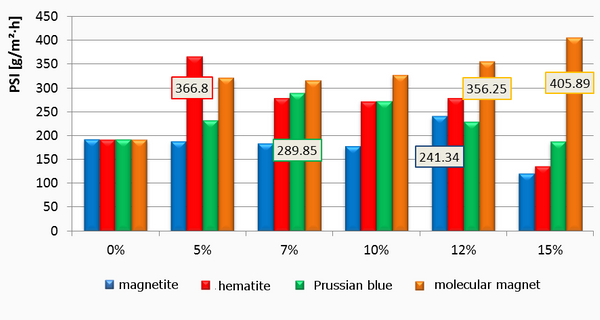
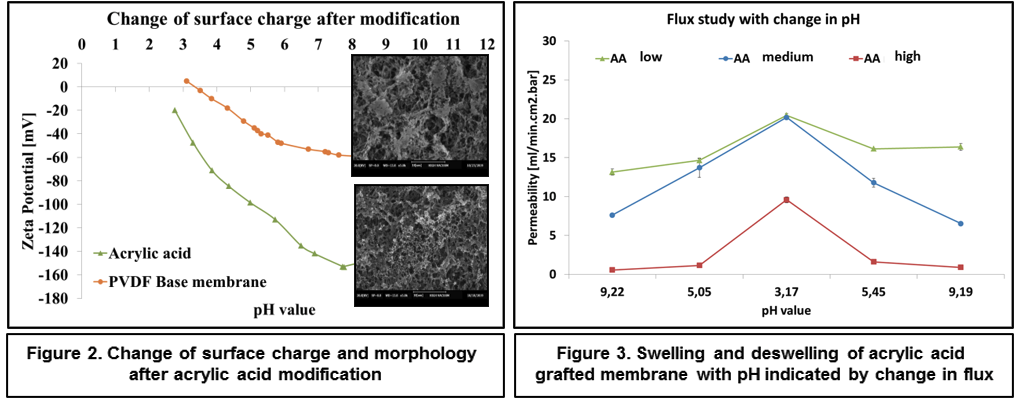
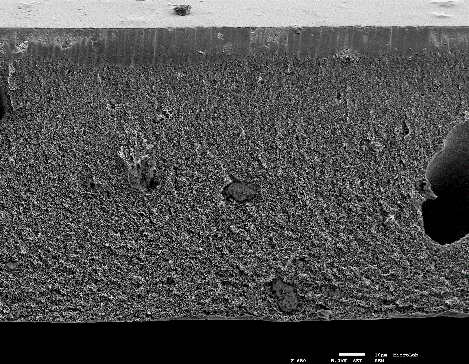
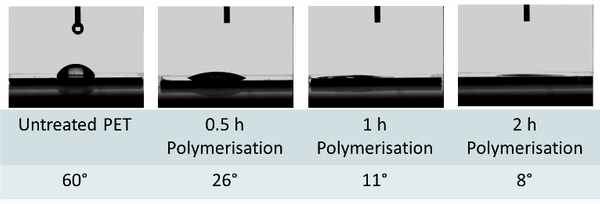
Successful functionalization of PVDF with different degrees of grafting and change in morphology were verified by Fourier Transform-Infrared spectroscopy and Scanning Electron Microscopy, respectively. The grafted membranes showed enhanced hydrophilicity (contact angle less than 10° from 110° before modification) and change in surface charge (Figure 2). Flux study provided evidence of reduced pore size of the grafted membrane with dependence on solution pH for acrylic acid-grafted membranes (Figure 3). The decrease in flux was potentially caused by the swelling of grafted hydrogel matrix. Interestingly, filtration results showed that grafted membranes have higher recovery of particles compared to the control membranes manufactured by blending of the polymer with a hydrophilic additive.
This study not only provided PVDF membranes with different surface properties potentially useful for various bio-medical applications, but also proposed a novel scalable functionalization approach to enhance the performance of advanced functionalized polymeric membranes.
Acknowledgements
The project has received funding from the European Union´s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 722148.


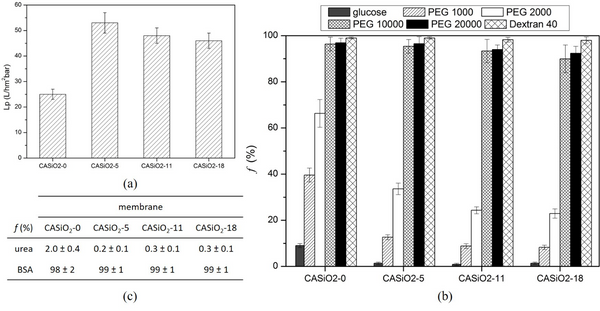
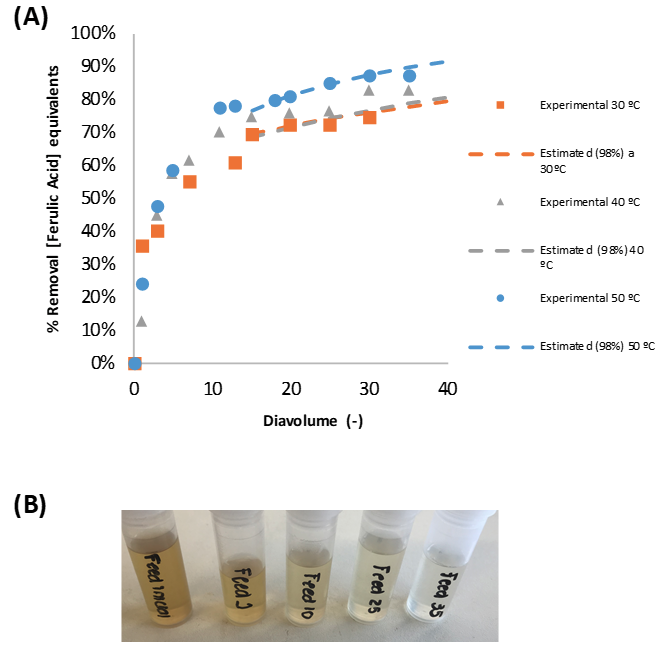
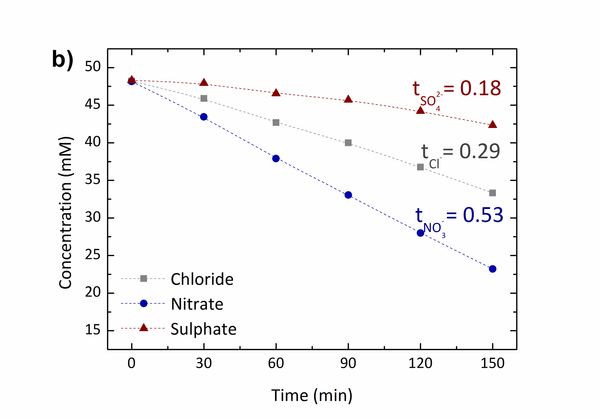
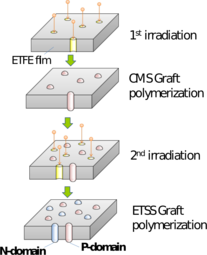
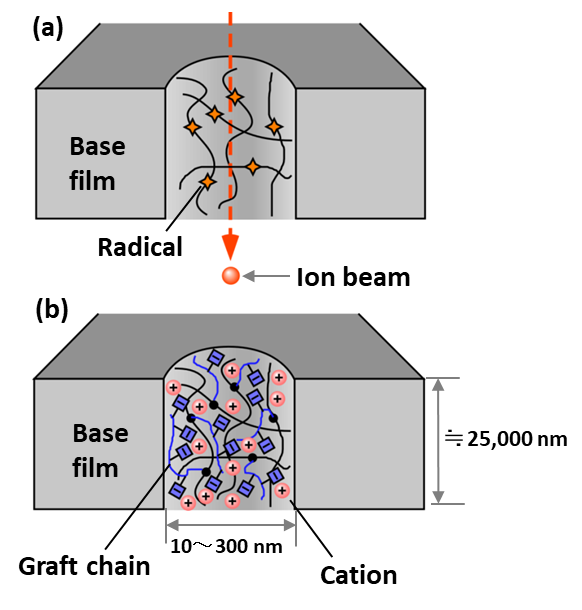 The obtained CEMs showed the ion exchange capacity (IEC) ranging from 0.38 to 1.70 mmol/g. The membrane resistance of the CEM with the IEC of 1.70 mmol/g was 0.18 Ω cm2, which was only around one tenth of that of a commercial CEM, Neosepta® CMX.
The obtained CEMs showed the ion exchange capacity (IEC) ranging from 0.38 to 1.70 mmol/g. The membrane resistance of the CEM with the IEC of 1.70 mmol/g was 0.18 Ω cm2, which was only around one tenth of that of a commercial CEM, Neosepta® CMX.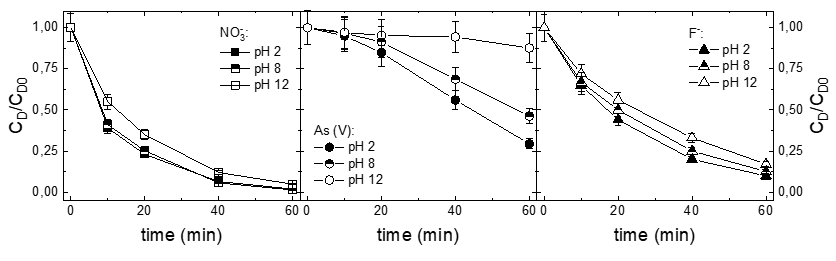
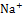 and
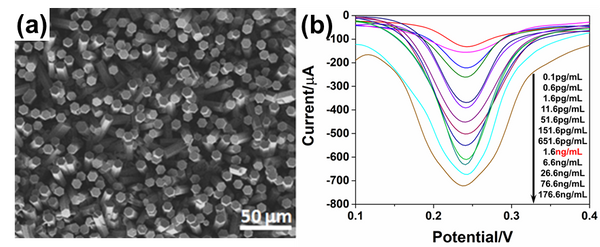
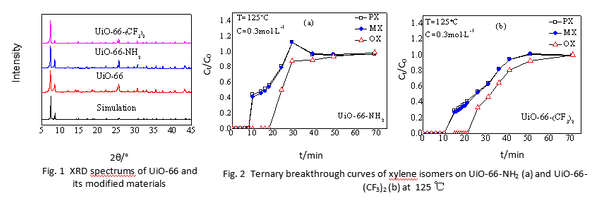
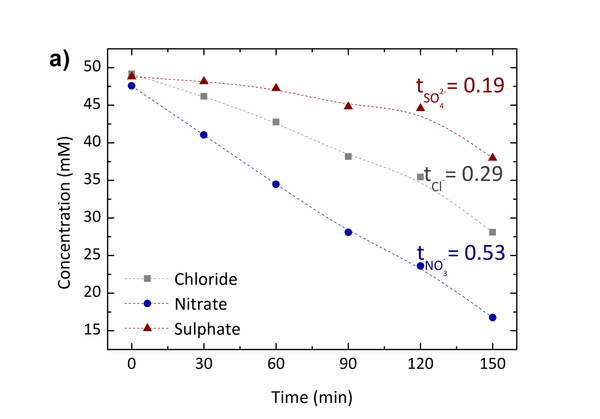
and 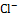 ), we use redox reaction of Zinc- iodine couple. During the desalination process, zinc anode consumes chloride ions to make zinc chlorides, and as a cathodic feed stream, sodium triiodide consumes sodium ions to make sodium iodides. We compared the I-V curves of fabricated devices using zinc as anode and using both zinc anode and sodium triiodide as cathodic feed stream. The overlimiting current regime is started at -0.4V and 0.2V respectively while normal ED is at 1.6V. Also with zinc-iodine coupled device, non linear ion concentration polarization is occurred at 0V and EC is clearly visualized at 0.5V. With this EC enhancement and operating voltage drop by chemical energy, the required energy per ion removal (EPIR) with 85% of salt removal ratio is only 65% compare to the typical ED system. These results show that this method is effective as a low energy method for desalination.
), we use redox reaction of Zinc- iodine couple. During the desalination process, zinc anode consumes chloride ions to make zinc chlorides, and as a cathodic feed stream, sodium triiodide consumes sodium ions to make sodium iodides. We compared the I-V curves of fabricated devices using zinc as anode and using both zinc anode and sodium triiodide as cathodic feed stream. The overlimiting current regime is started at -0.4V and 0.2V respectively while normal ED is at 1.6V. Also with zinc-iodine coupled device, non linear ion concentration polarization is occurred at 0V and EC is clearly visualized at 0.5V. With this EC enhancement and operating voltage drop by chemical energy, the required energy per ion removal (EPIR) with 85% of salt removal ratio is only 65% compare to the typical ED system. These results show that this method is effective as a low energy method for desalination.