Category
Video Presentation Link.
Enter your abstract or case.
Introduction
Brugada Syndrome (BrS) is a rare arrhythmogenic entity that poses a risk of sudden cardiac death (SCD) due to ventricular arrhythmias. Sinus node dysfunction (SND) is a less recognized and acknowledged conduction abnormality associated with BrS, but nevertheless an important potential manifestation that can alter management.
Case description
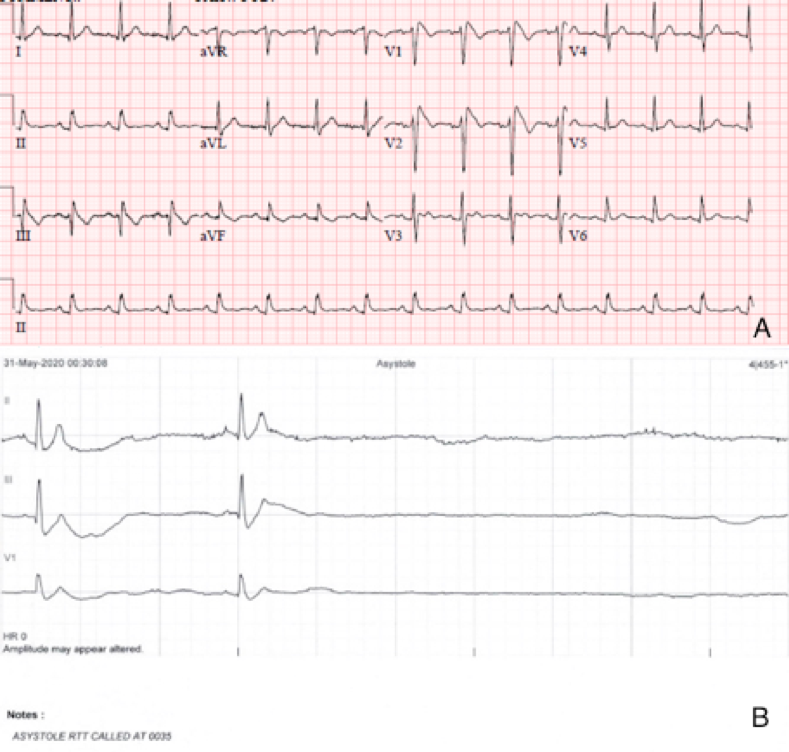
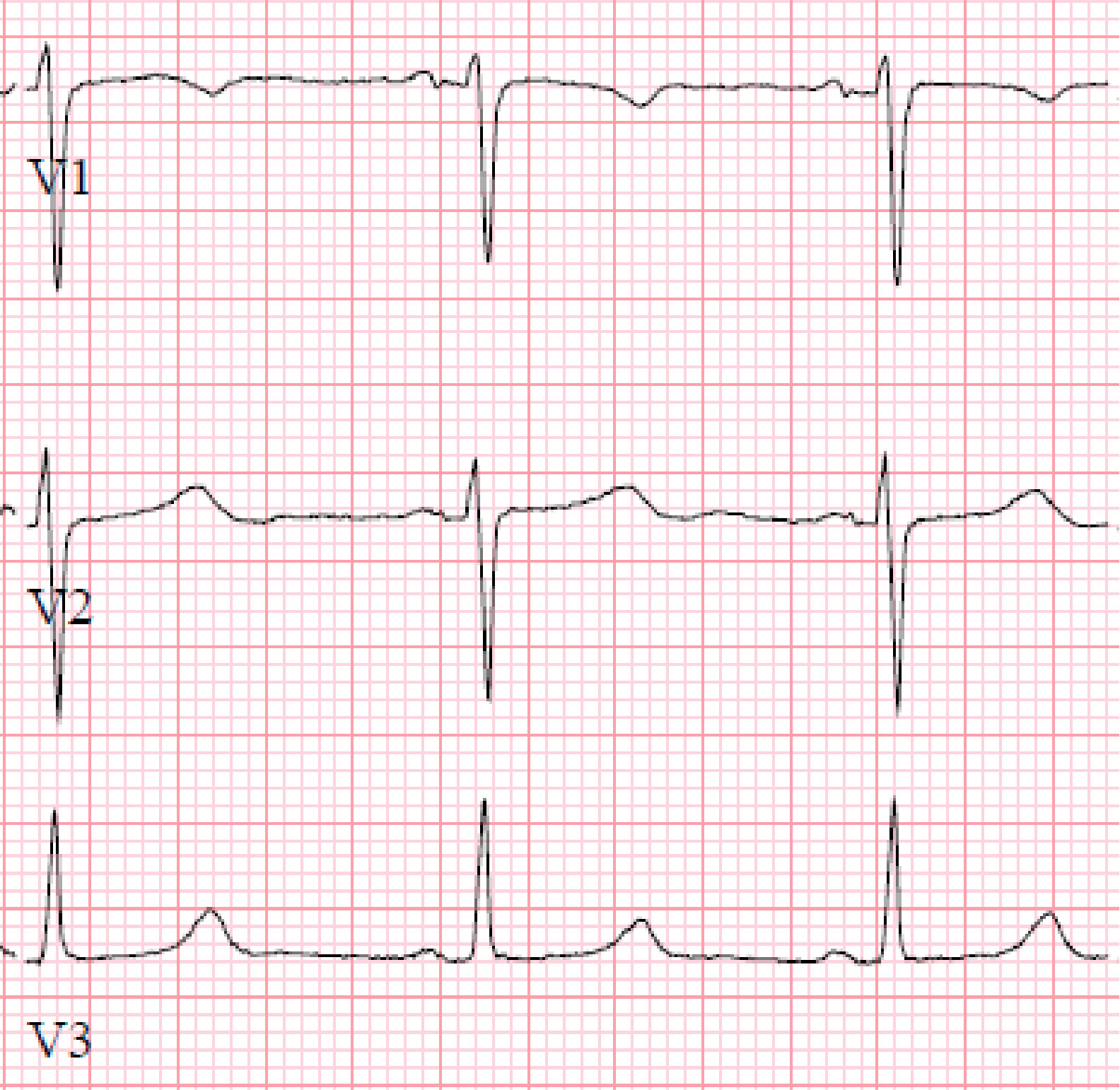
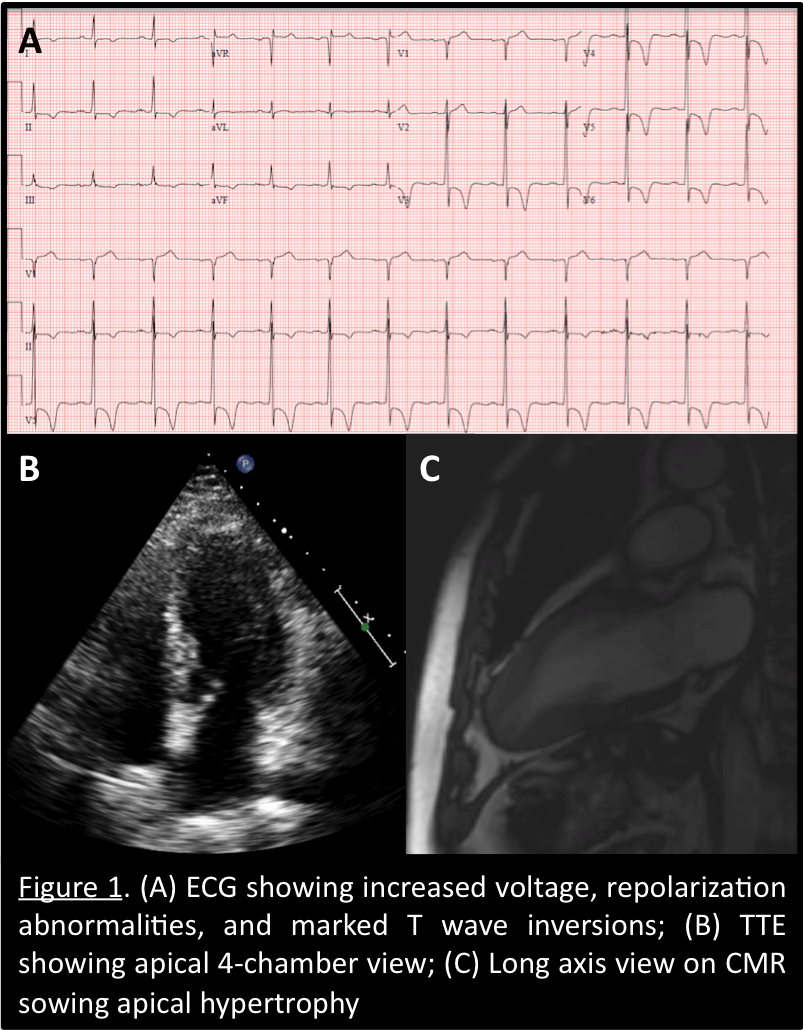
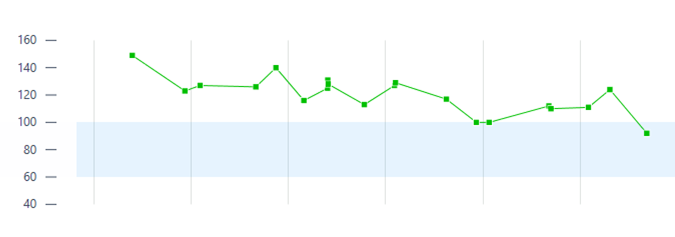
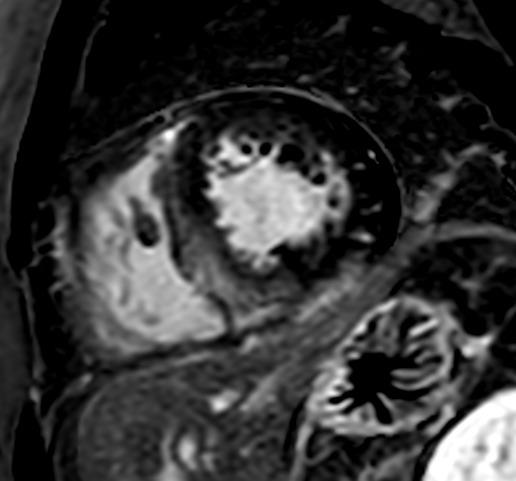
This is a case of a 31-year-old male presenting with syncope and an ECG consistent with type-1 Brugada pattern (Figure-1-A). His workup including an echocardiogram was unremarkable. Due to the concern for Brugada associated ventricular tachyarrhythmias as the culprit for his syncopal event, the placement of a defibrillator was recommended. The patient elected to have a subcutaneous defibrillator (S-ICD) due to its cosmetic advantage, lack of vascular injury risk and low systemic infection risk. Unfortunately, after placement he had another inpatient syncopal episode associated with dizziness secondary to a 12 second sinus arrest documented on telemetry monitoring (Figure-1-B). Continuous monitoring revealed multiple more episodes of sinus pauses of different durations. Given the concern that his presenting syncope was a result of sinus arrest rather than the presumed ventricular arrythmia that is classically associated with Brugada, the patient was presented with different management options including the placement of a pacemaker or a dual chamber ICD and the future removal of the S-ICD. He deferred further procedures and elected to pursue outpatient cardiac event monitoring.
Discussion
Syncope or SCD in patients with BrS is classically attributed to ventricular arrhythmias.1 SND in patients with BrS has been reported in the literature; but remains clinically a less recognized presentation. Fatty tissue deposition, atrophy and fibrosis of the sinus node was illustrated on autopsy of a young patient with BrS who died suddenly.2 In a study of 59 asymptomatic patients with Brugada, 17% demonstrated SND induced during electrophysiology study.3 Clinically documented SND in patients with BrS was estimated to be 1.1-1.7% in large population studies.4,5 A possible bradycardic mode of death was suggested with a carrier of the a mutant SCN5A gene, who suffered from sinus arrest > 9 seconds.6
A mutation in the cardiac predominant sodium channel α-subunit SCN5A which accounts for 20- 30% of patients with Brugada, has been linked to SND.7, 8, 9, 10 The abnormal sodium channel is thought to prolong the action potential of sinus node cells and slow depolarization.6 As a result, an overlap syndrome of cardiac sodium channelopathy including BrS, conduction disease and long QT syndrome has been proposed.11,12 Unfortunately, SND in our patient was apparent after his S-ICD was already placed for a presumed ventricular arrhythmogenic etiology of his presenting syncope.
Conclusion
It is imperative that providers and cardiologists are aware of the association of SND with BrS which is likely related to common genetic mutations leading to an overlap syndrome. We recommend avoiding the placement of S-ICD which may necessitate future procedures such as the placement of a pacemaker or transvenous ICD and the removal of S-ICD in the event that SND is to develop or is revealed as the etiology of a syncopal event.
Figure1-A-ECG shows Brugada pattern; B-Telemetry strip reveals sinus arrest.