Abstract Text
The structure and biological functions of essential biomolecules such as proteins and nucleic acids are driven by electrostatic interactions, which in turn crucially depend on the dielectric polarization properties of the interacting molecules and the water molecules that surround them. Knowledge of these properties is therefore of paramount importance to get an insight into biomolecular structure, dynamics and hydration [1]. However, probing the dielectric response of biomolecules on a molecular scale has traditionally been a technical challenge due to the lack of tools with sufficient sensitivity. In recent years, great advances have been made based on the use of a novel scanning probe microscopy technique, scanning dielectric microscopy, which allowed researchers to probe the dielectric constants of DNA condensed inside single viruses [2,3], lipid biolayers [4,5] and confined water [6], amongst others. Here, we present our more recent advances in which we applied this technique to single self-assembled I3K peptide chains and monolayers of lysozyme proteins in aqueous solution. We show that, by using a conductive AFM tip and substrate as electrodes, the capacitive response of the molecules to the applied voltage can be measured directly on the molecular scale at MHz frequencies. The dielectric polarization properties of the molecules are then extracted from the measured capacitive interaction by means of detailed finite-element calculations. This work is another important step towards our understanding of dielectric properties of biomolecules on the molecular scale and it offers much needed feedback for theories describing electrostatic interactions in biology.
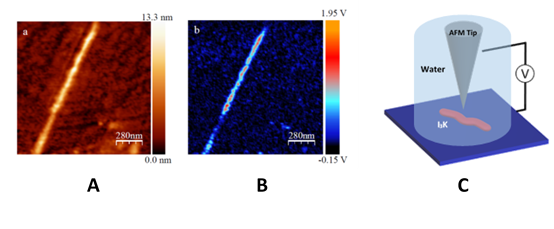
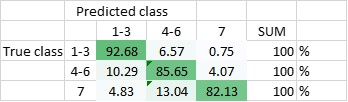
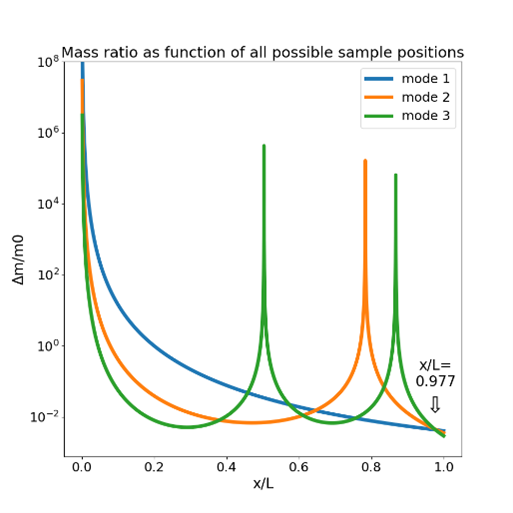
Figure 1 (a,b) Topography and corresponding dielectric image of an I3K peptide chain adsorbed onto a silicon wafer taken in aqueous solution. Dielectric contrast is clearly detected over the peptide chain. (c) Simplified schematic of the tip-sample system under study using the in-liquid scanning dielectric microscopy.
Keywords
Scanning Probe Microscopy, Atomic Force Microscopy, Scanning Dielectric Microscopy, Peptides, Proteins, Dielectric polarisation properties
References
[1] B. Honig, A. Nicholls, Science 268, 1144 (1995).
[2] L. Fumagalli, D. Esteban, A. Cuervo, J.L. Carrascosa, G. Gomila Nature Mater. 11, 808 (2012)
[3] A. Cuervo, P.D. Dans, J.L. Carrascosa, G. Gomila, L. Fumagalli PNAS, 111, 362 (2014)
[4] G. Gramse, M. A. Edwards, L. Fumagalli, G. Gomila Appl. Phys. Lett 101, 213108 (2012)
[5] A. Dols-Perez, G. Gramse, A. Calò, G. Gomila, L. Fumagalli Nanoscale 7, 1832 (2015)
[6] L. Fumagalli, A. Esfandiar, R. Fabregas et al. Science 360, 1339 (2018)