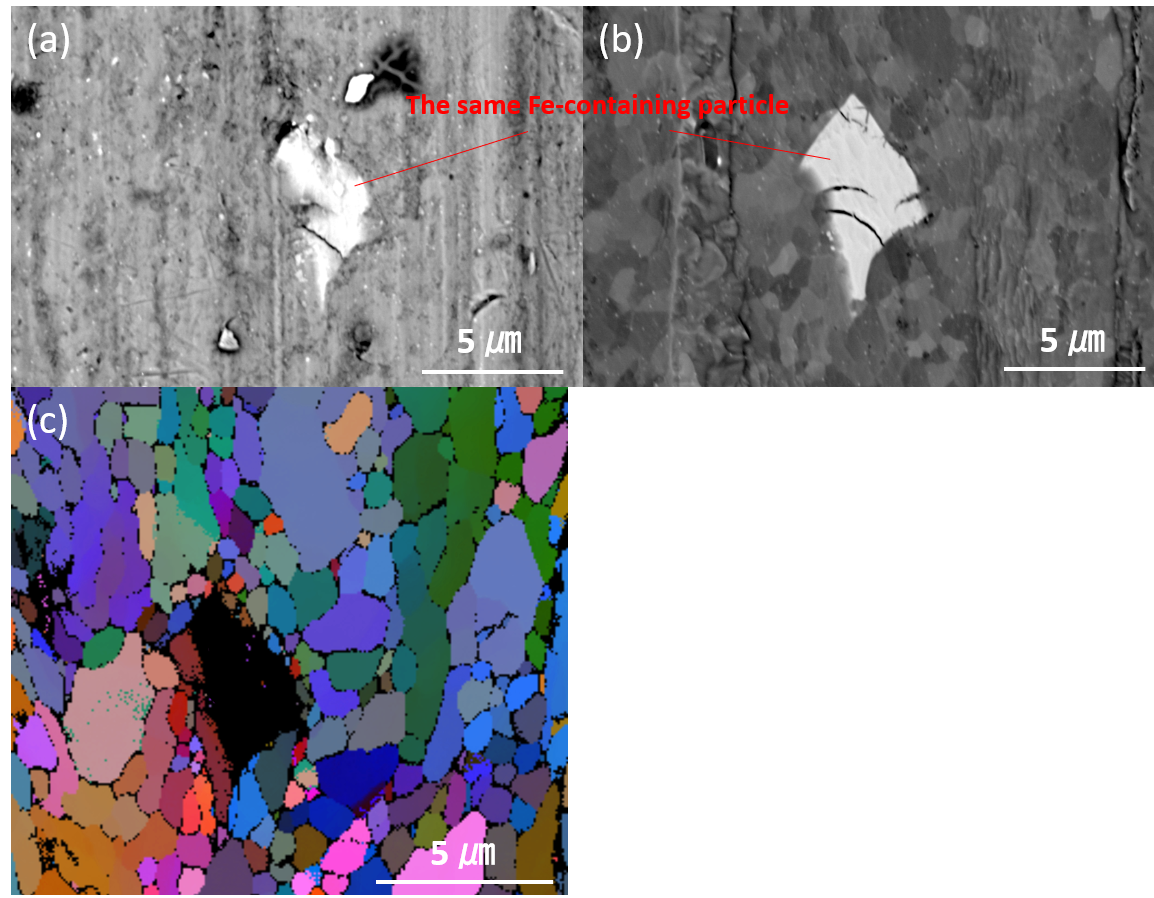
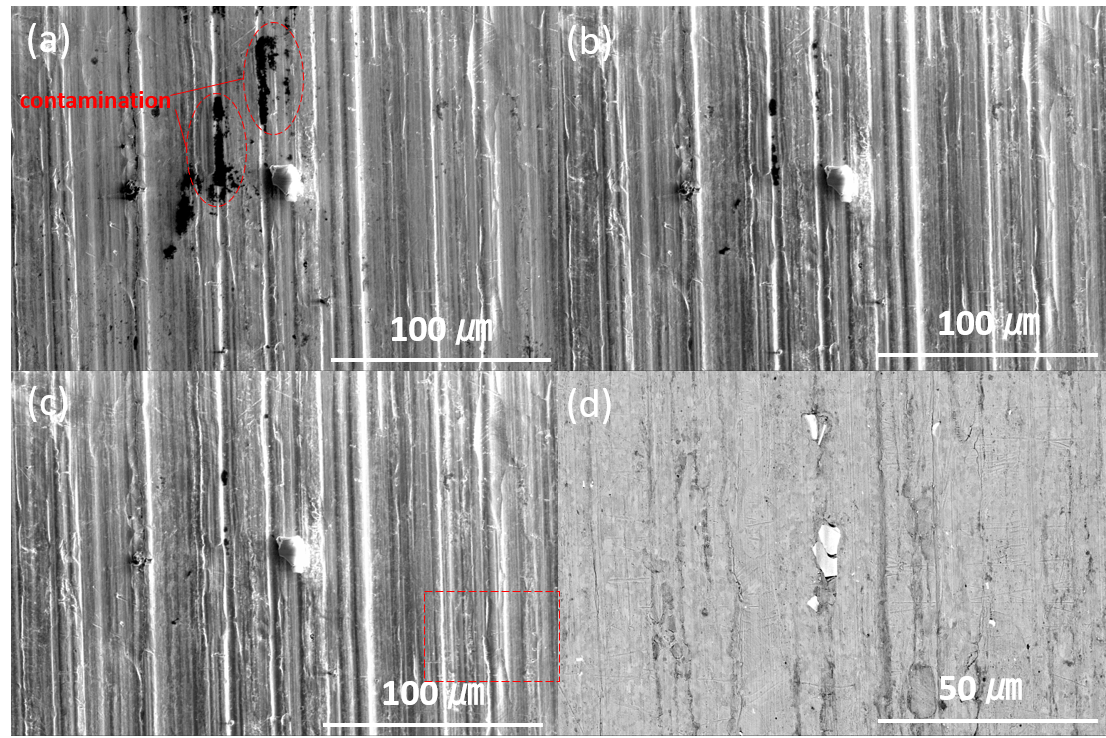
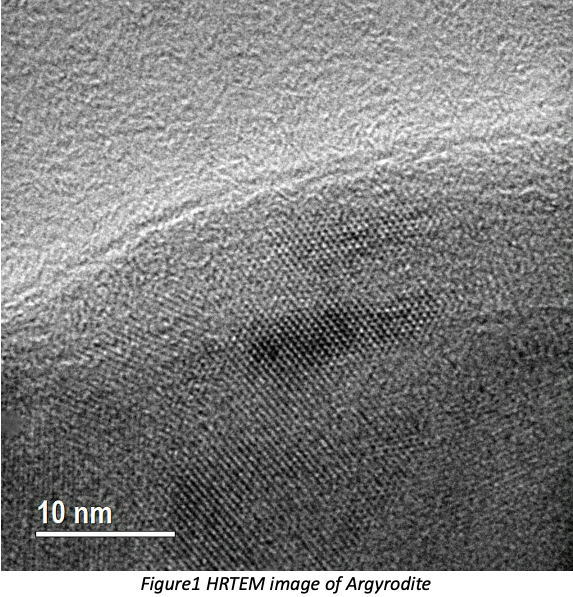
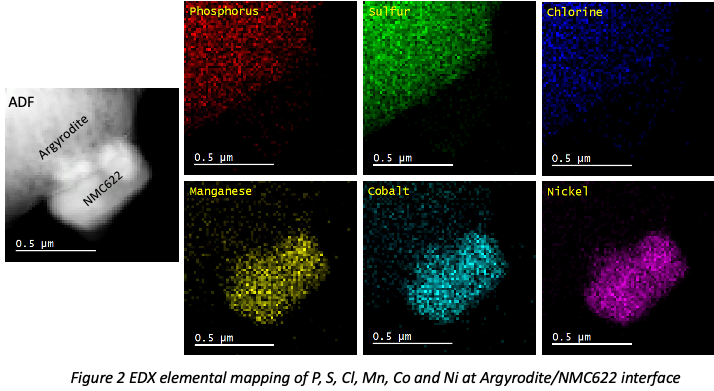
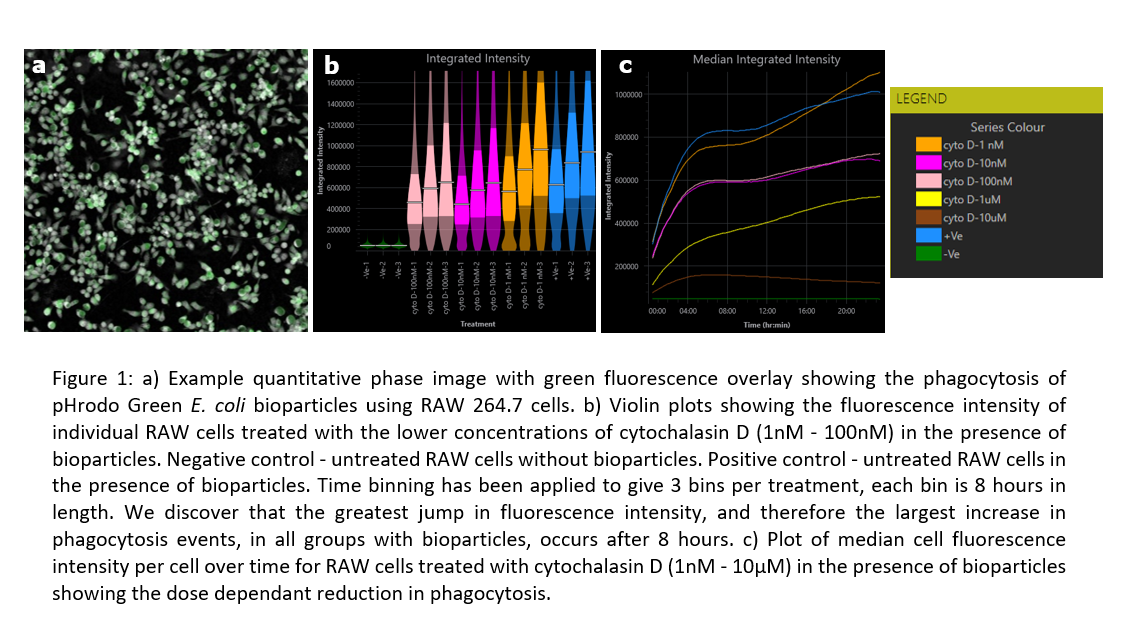
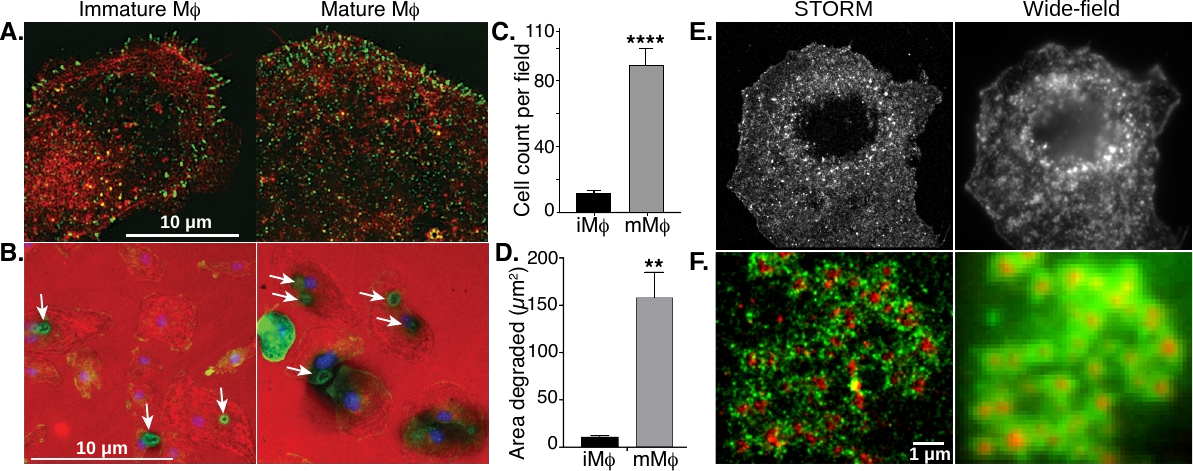
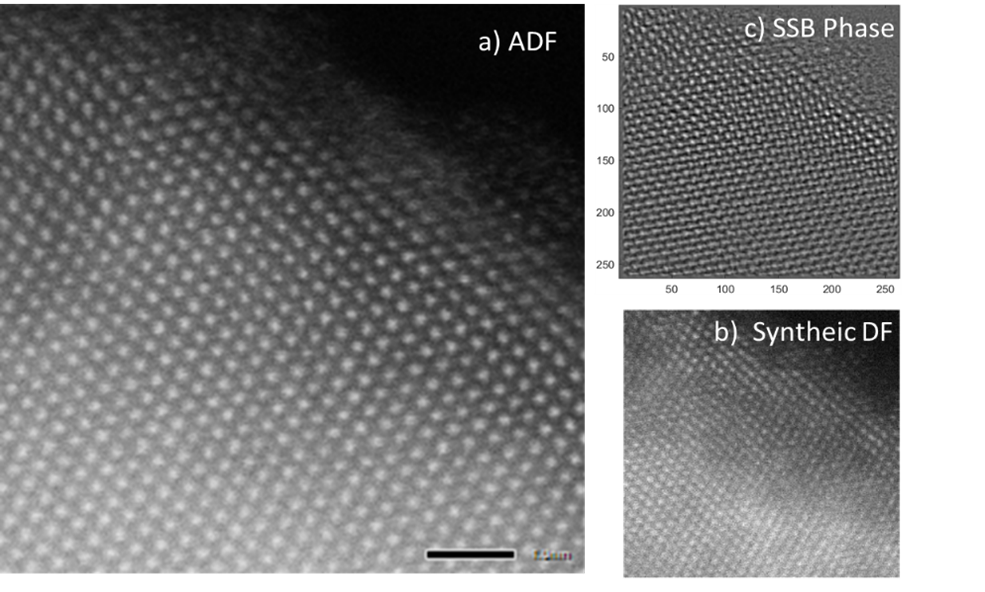
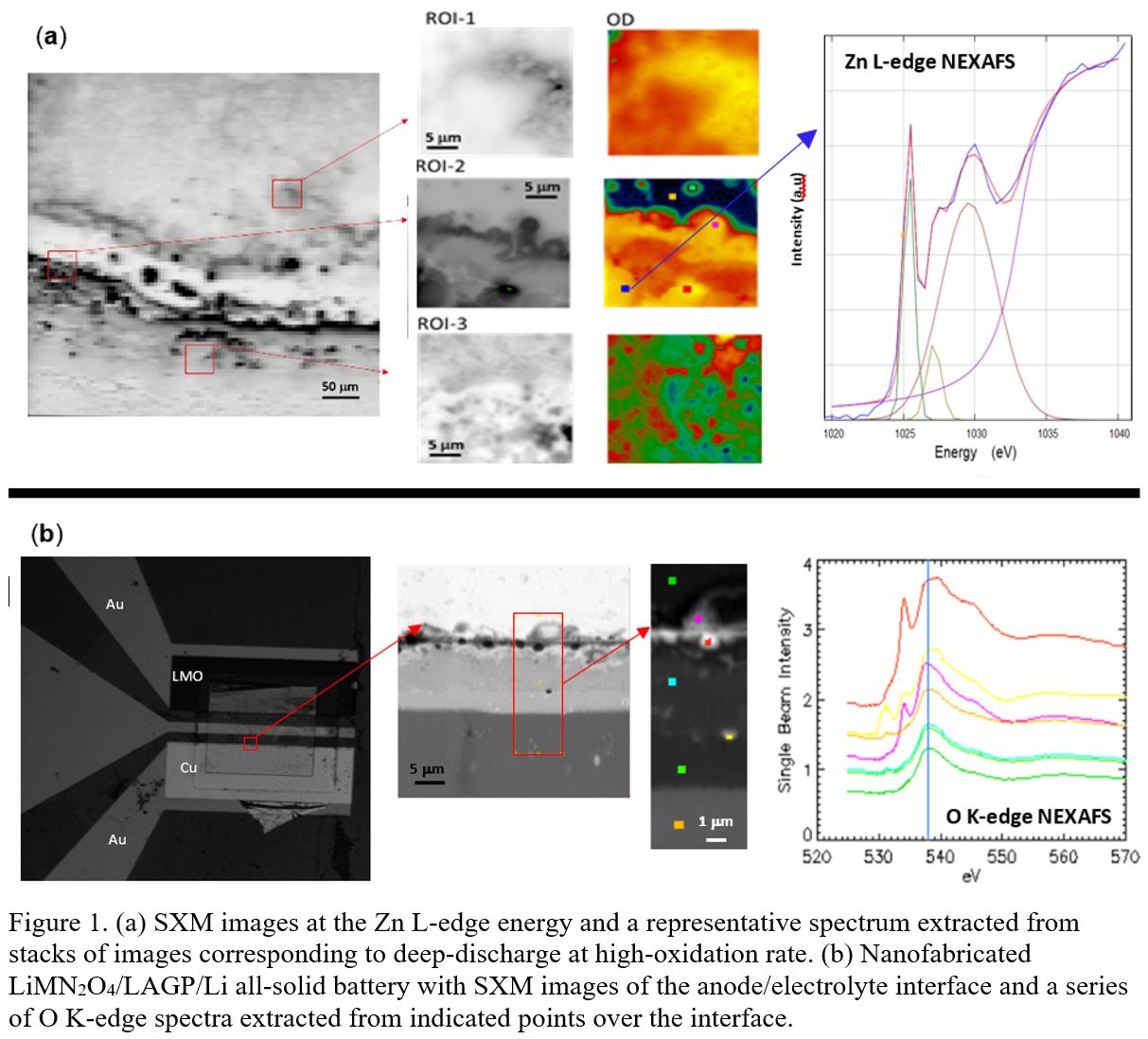
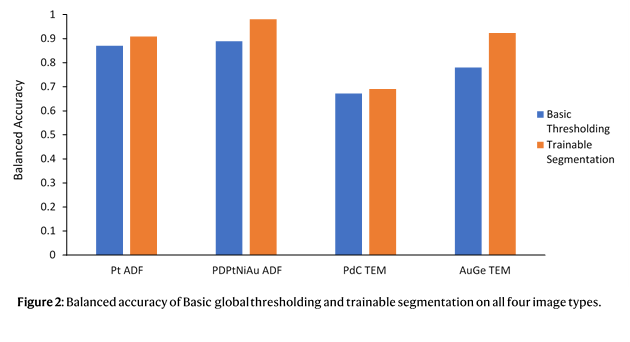
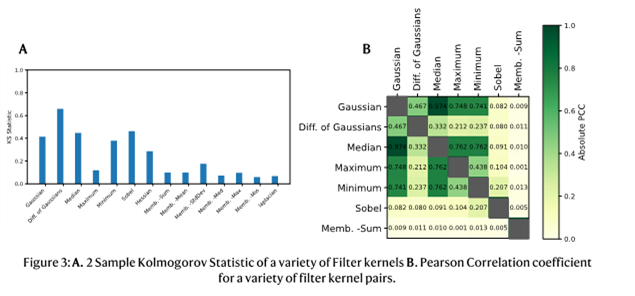
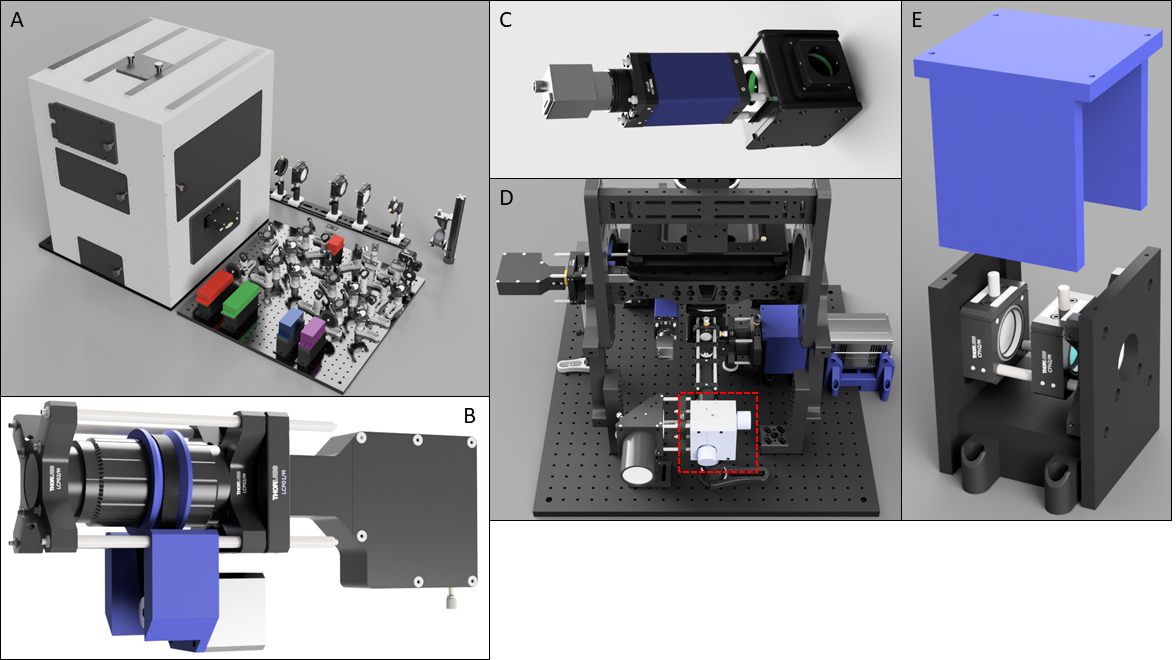
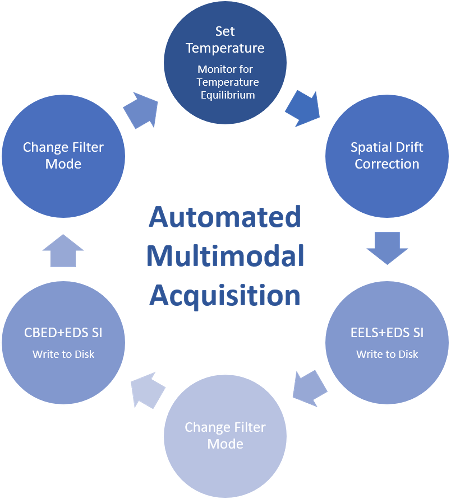
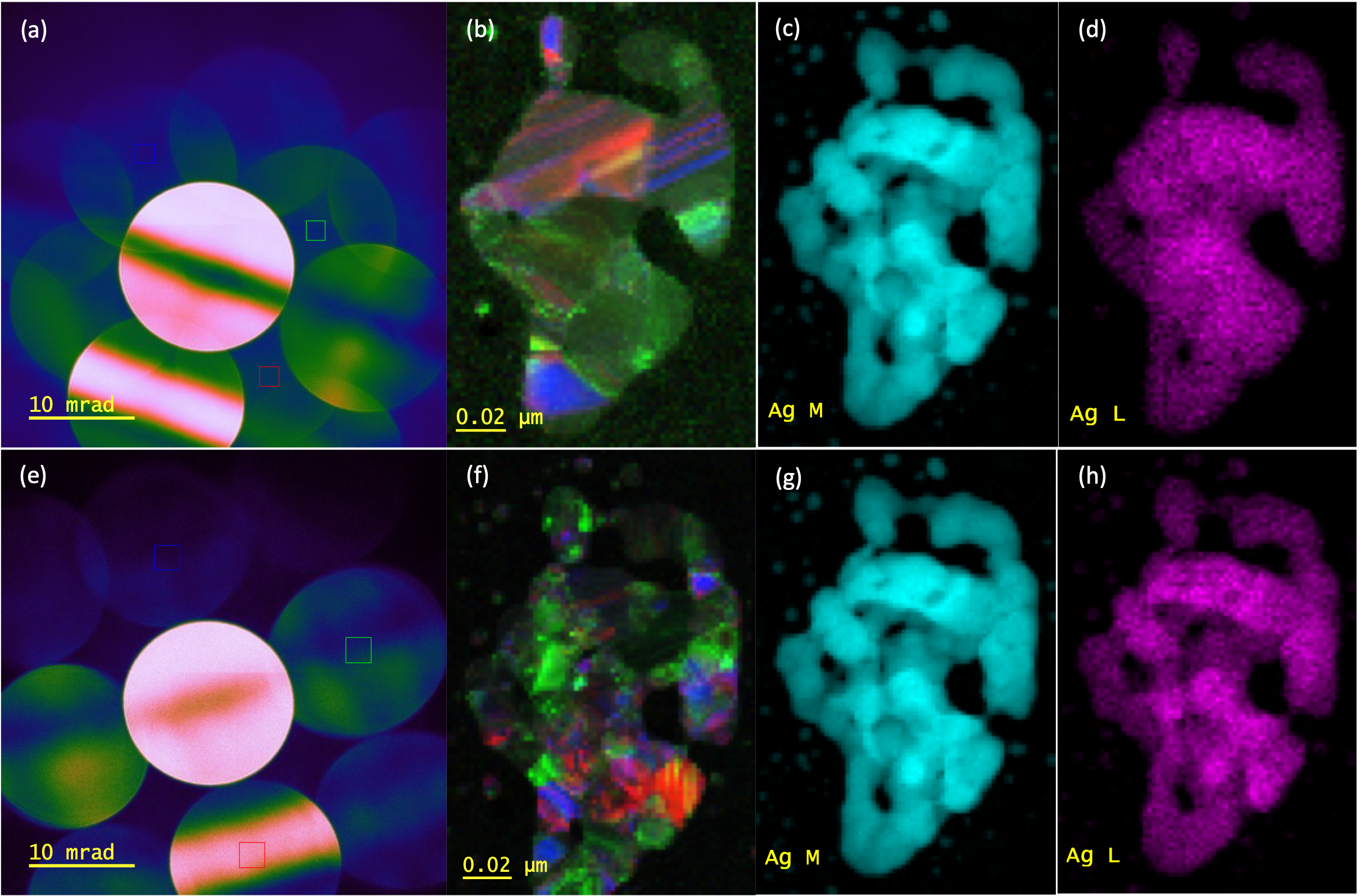
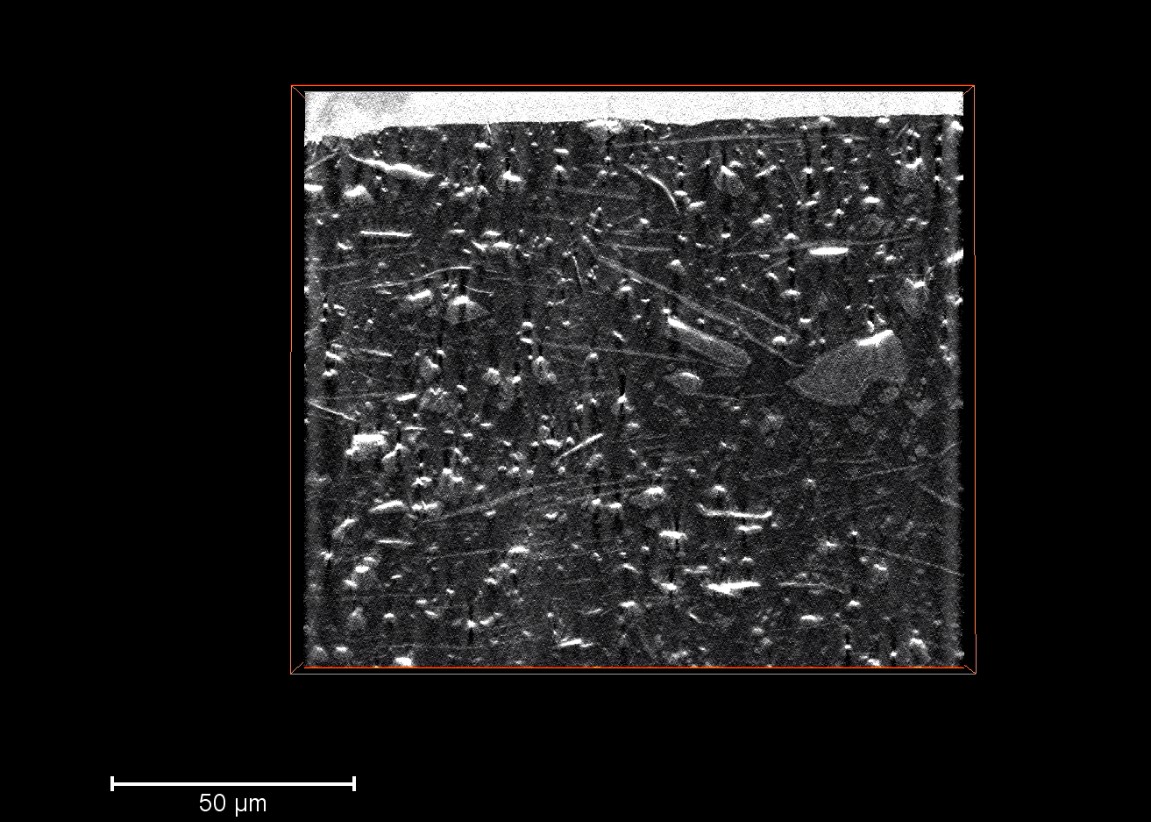
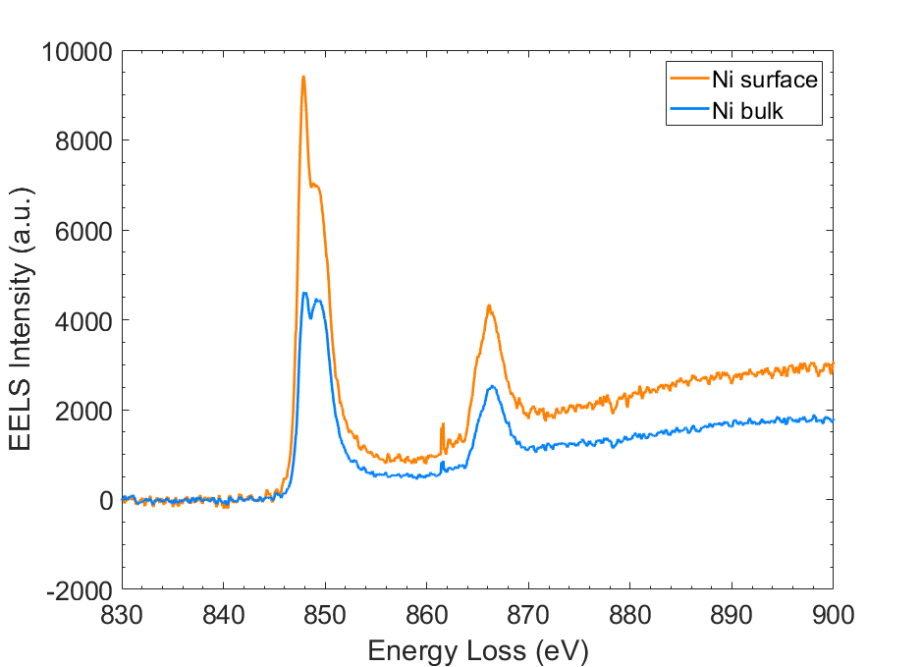
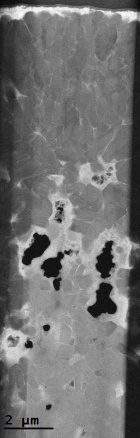
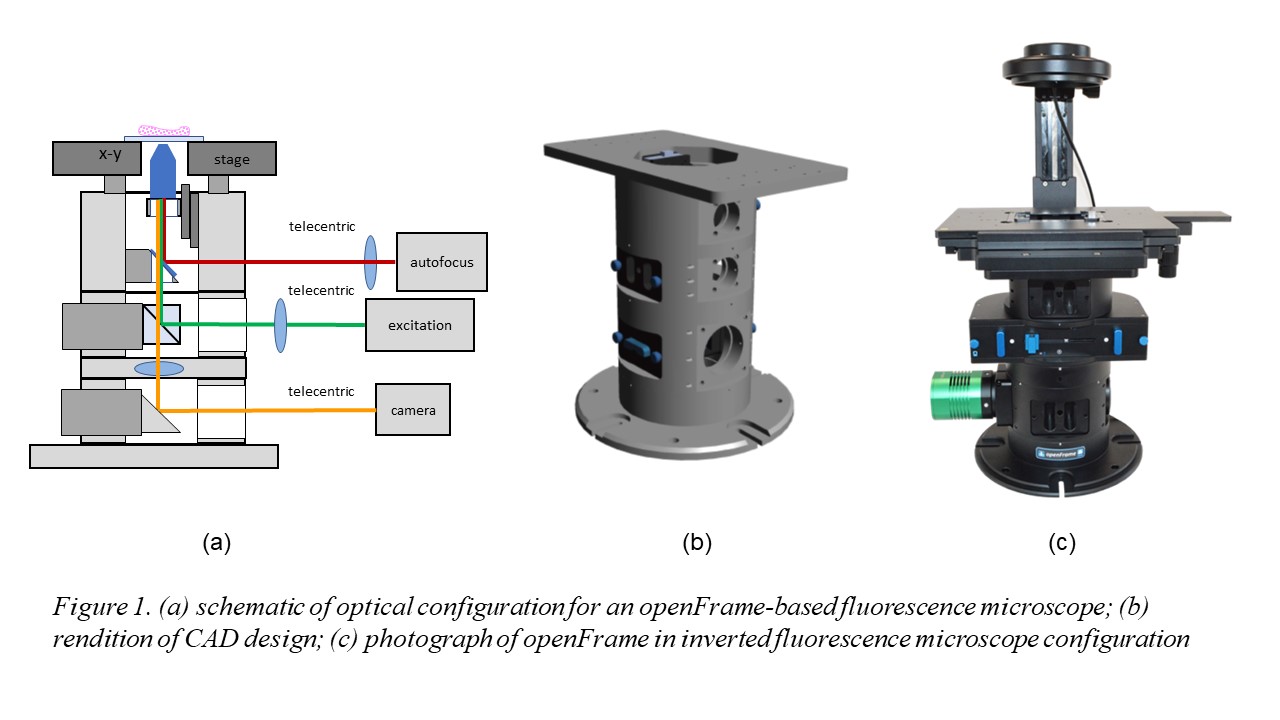
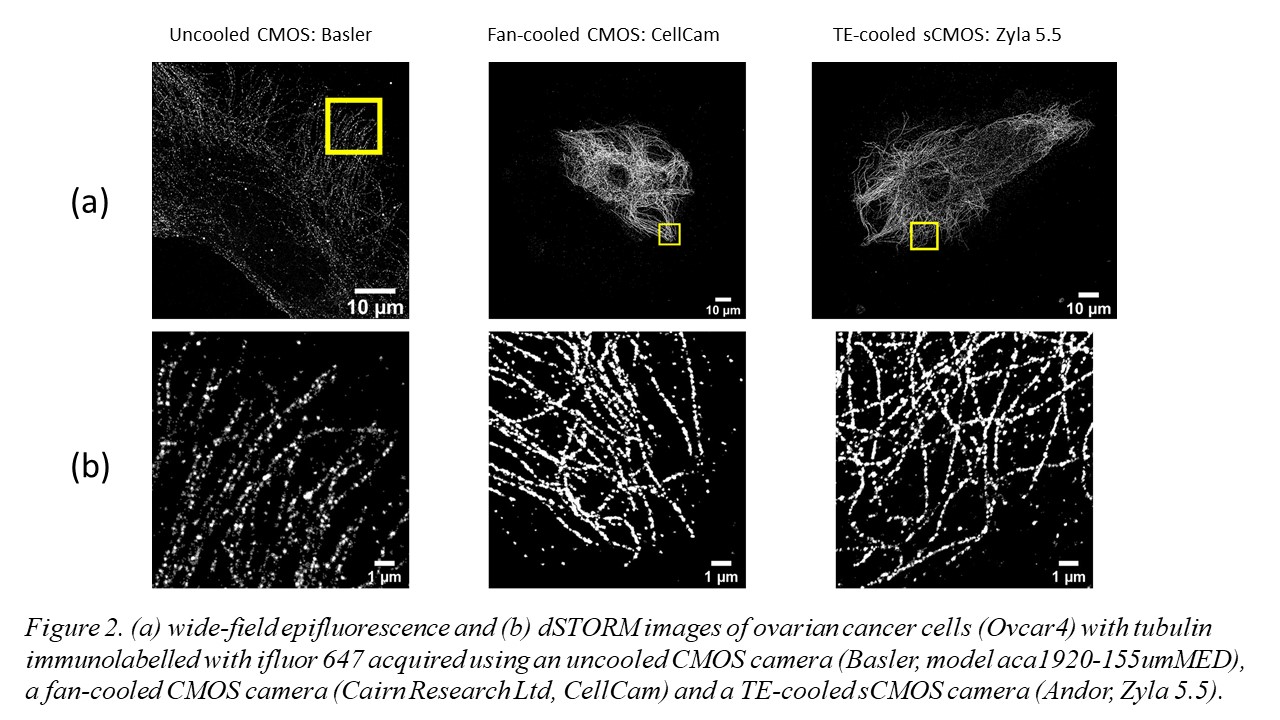
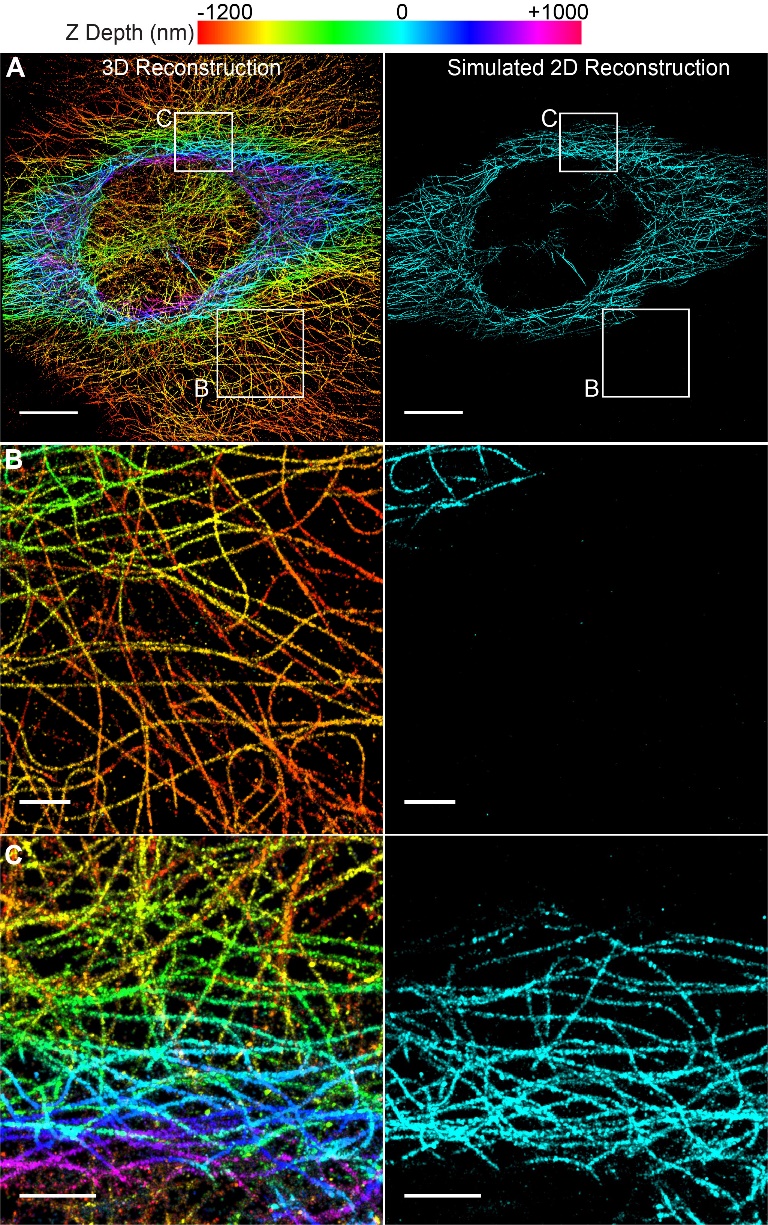
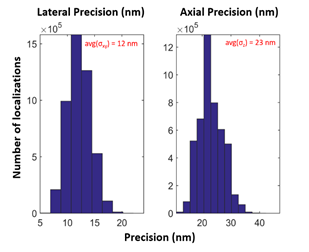
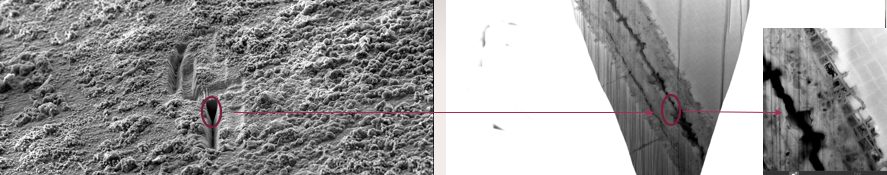
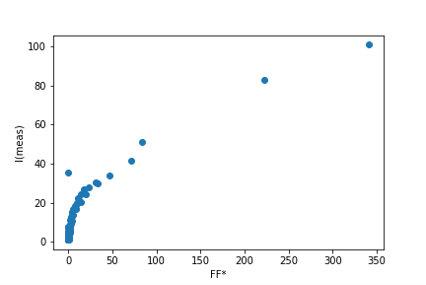
1. Liu, C.; Neale, Z. G.; Cao, G., Understanding electrochemical potentials of cathode materials in rechargeable batteries. Materials Today 2016, 19 (2), 109-123.
2. Lain; Brandon; Kendrick, Design Strategies for High Power vs. High Energy Lithium Ion Cells. Batteries 2019, 5 (4).
3. Yue, Y.; Liang, H., Micro- and Nano-Structured Vanadium Pentoxide (V2O5) for Electrodes of Lithium-Ion Batteries. Advanced Energy Materials 2017, 7 (17), 1602545.
4. N., N.; F., W.; T., L. J.; G., Y., Li-ion battery materials: Present and future. 2015, 18, 252.
5. Stringer, J., The vanadium-oxygen system—A review. Journal of the Less Common Metals 1965, 8 (1), 1-14.
6. Kang, Y.-B., Critical evaluation and thermodynamic optimization of the VO–VO2. 5 system. Journal of the European Ceramic Society 2012, 32 (12), 3187-3198.
7. Wang, L.; Huang, K. W.; Chen, J.; Zheng, J., Ultralong cycle stability of aqueous zinc-ion batteries with zinc vanadium oxide cathodes. Sci Adv 2019, 5 (10), eaax4279.
8. Gu, S.; Wang, H.; Wu, C.; Bai, Y.; Li, H.; Wu, F., Confirming reversible Al 3+ storage mechanism through intercalation of Al 3+ into V 2 O 5 nanowires in a rechargeable aluminum battery. Energy Storage Materials 2017, 6, 9-17.
9. Yoo, H. D.; Jokisaari, J. R.; Yu, Y.-S.; Kwon, B. J.; Hu, L.; Kim, S.; Han, S.-D.; Lopez, M.; Lapidus, S. H.; Nolis, G. M.; Ingram, B. J.; Bolotin, I.; Ahmed, S.; Klie, R. F.; Vaughey, J. T.; Fister, T. T.; Cabana, J., Intercalation of Magnesium into a Layered Vanadium Oxide with High Capacity. ACS Energy Letters 2019, 4 (7), 1528-1534.
10. Delmas, C.; Brethes, S.; Menetrier, M., ω-LixV2O5—a new electrode material for rechargeable lithium batteries. Journal of power sources 1991, 34 (2), 113-118.
11. Cocciantelli, J.; Menetrier, M.; Delmas, C.; Doumerc, J.; Pouchard, M.; Broussely, M.; Labat, J., On the δ→ γ irreversible transformation in Li//V2O5 secondary batteries. Solid State Ionics 1995, 78 (1-2), 143-150.
12. Sides, C. R.; Martin, C. R., Nanostructured Electrodes and the Low‐Temperature Performance of Li‐Ion Batteries. Advanced Materials 2005, 17 (1), 125-128.
13. Chan, C. K.; Peng, H.; Twesten, R. D.; Jarausch, K.; Zhang, X. F.; Cui, Y., Fast, completely reversible Li insertion in vanadium pentoxide nanoribbons. Nano letters 2007, 7 (2), 490-495.
14. De Jesus, L. R.; Horrocks, G. A.; Liang, Y.; Parija, A.; Jaye, C.; Wangoh, L.; Wang, J.; Fischer, D. A.; Piper, L. F.; Prendergast, D., Mapping polaronic states and lithiation gradients in individual V 2 O 5 nanowires. Nature communications 2016, 7 (1), 1-9.
15. Lee, J.-K.; Kim, G.-P.; Song, I. K.; Baeck, S.-H., Electrodeposition of mesoporous V2O5 with enhanced lithium-ion intercalation property. Electrochemistry communications 2009, 11 (8), 1571-1574.
16. Huo, D.; Contreras, A.; Laïk, B.; Bonnet, P.; Guérin, K.; Muller-Bouvet, D.; Cenac-Morthe, C.; Baddour-Hadjean, R.; Pereira-Ramos, J.-P., Evidence for a nanosize effect on the structural and high performance electrochemical properties of V2O5 obtained via fluorine chemistry. Electrochimica Acta 2017, 245, 350-360.
17. Pu, J.; Shen, Z.; Zhong, C.; Zhou, Q.; Liu, J.; Zhu, J.; Zhang, H., Electrodeposition Technologies for Li‐Based Batteries: New Frontiers of Energy Storage. Advanced Materials 2019, 1903808.
18. Meulenkamp, E. A.; van Klinken, W.; Schlatmann, A. R., In-situ X-ray diffraction of Li intercalation in sol–gel V2O5 films. Solid State Ionics 1999, 126 (3), 235-244.
19. Zhong, W.; Huang, J.; Liang, S.; Liu, J.; Li, Y.; Cai, G.; Jiang, Y.; Liu, J., New Prelithiated V2O5 Superstructure for Lithium-Ion Batteries with Long Cycle Life and High Power. ACS Energy Letters 2019, 5 (1), 31-38.
20. Liu, H.; Zhu, Z.; Yan, Q.; Yu, S.; He, X.; Chen, Y.; Zhang, R.; Ma, L.; Liu, T.; Li, M.; Lin, R.; Chen, Y.; Li, Y.; Xing, X.; Choi, Y.; Gao, L.; Cho, H. S.; An, K.; Feng, J.; Kostecki, R.; Amine, K.; Wu, T.; Lu, J.; Xin, H. L.; Ong, S. P.; Liu, P., A disordered rock salt anode for fast-charging lithium-ion batteries. Nature 2020, 585 (7823), 63-67.
21. Lu, Y.; Zhou, X., Synthesis and characterization of nanorod-structured vanadium oxides. Thin Solid Films 2018, 660, 180-185.
22. Lee, Y., The Effect of Active Material, Conductive Additives, and Binder in a Cathode Composite Electrode on Battery Performance. Energies 2019, 12 (4).
23. Delmas, C.; Cognac-Auradou, H.; Cocciantelli, J. M.; Ménétrier, M.; Doumerc, J. P., The LixV2O5 system: An overview of the structure modifications induced by the lithium intercalation. Solid State Ionics 1994, 69 (3), 257-264.
24. Galy, J., Vanadium pentoxide and vanadium oxide bronzes—Structural chemistry of single (S) and double (D) layer MxV2O5 phases. Journal of Solid State Chemistry 1992, 100 (2), 229-245.
25. Marley, P. M.; Horrocks, G. A.; Pelcher, K. E.; Banerjee, S., Transformers: the changing phases of low-dimensional vanadium oxide bronzes. Chemical Communications 2015, 51 (25), 5181-5198.
26. Christensen, C. K.; Sørensen, D. R.; Hvam, J.; Ravnsbæk, D. B., Structural Evolution of Disordered Li x V2O5 Bronzes in V2O5 Cathodes for Li-Ion Batteries. Chemistry of Materials 2018, 31 (2), 512-520.
27. Delmas, C.; Brèthes, S.; Ménétrier, M., ω-LixV2O5 — a new electrode material for rechargeable lithium batteries. Journal of Power Sources 1991, 34 (2), 113-118.
28. Zhu, J.; Shen, H.; Shi, X.; Yang, F.; Hu, X.; Zhou, W.; Yang, H.; Gu, M., Revealing the Chemical and Structural Evolution of V2O5 Nanoribbons in Lithium-Ion Batteries Using in Situ Transmission Electron Microscopy. Analytical chemistry 2019, 91 (17), 11055-11062.
29. Mukherjee, A.; Sa, N.; Phillips, P. J.; Burrell, A.; Vaughey, J.; Klie, R. F., Direct Investigation of Mg Intercalation into the Orthorhombic V2O5 Cathode Using Atomic-Resolution Transmission Electron Microscopy. Chemistry of Materials 2017, 29 (5), 2218-2226.
30. Mukherjee, A.; Ardakani, H. A.; Yi, T.; Cabana, J.; Shahbazian-Yassar, R.; Klie, R. F., Direct characterization of the Li intercalation mechanism into α-V2O5 nanowires using in-situ transmission electron microscopy. Applied Physics Letters 2017, 110 (21), 213903.
31. Mukherjee, A.; Asayesh Ardakani, H.; Phillips, P. J.; Yassar, R. S.; Klie, R. F., Investigation of Li ion and Multivalent Battery Systems Using In situ TEM and High Resolution EELS. Microscopy and Microanalysis 2015, 21(S3), 1819-1820.
32. Hussein, H. E. M.; Maurer, R. J.; Amari, H.; Peters, J. J. P.; Meng, L.; Beanland, R.; Newton, M. E.; Macpherson, J. V., Tracking Metal Electrodeposition Dynamics from Nucleation and Growth of a Single Atom to a Crystalline Nanoparticle. ACS Nano 2018, 12 (7), 7388-7396.
33. Lim, J.; Li, Y.; Alsem, D. H.; So, H.; Lee, S. C.; Bai, P.; Cogswell, D. A.; Liu, X.; Jin, N.; Yu, Y. S.; Salmon, N. J.; Shapiro, D. A.; Bazant, M. Z.; Tyliszczak, T.; Chueh, W. C., Origin and hysteresis of lithium compositional spatiodynamics within battery primary particles. Science 2016, 353 (6299), 566-71.
34. Macpherson, J. V., A Practical Guide to Using Boron Doped Diamond in Electrochemical Research. Physical Chemistry Chemical Physics 2015, 17 (5), 2935-2949.
35. Hussein, H. E. M.; Amari, H.; Macpherson, J. V., Electrochemical Synthesis of Nanoporous Platinum Nanoparticles Using Laser Pulse Heating: Application to Methanol Oxidation. ACS Catalysis 2017, 7 (10), 7388-7398.
36. Hussein, H. E. M.; Ray, A. D.; Macpherson, J. V., Switching on palladium catalyst electrochemical removal from a palladium acetate–acetonitrile system via trace water addition. Green Chemistry 2019, 21 (17), 4662-4672.
37. Hussein, H. E. M.; Amari, H.; Breeze, B. G.; Beanland, R.; Macpherson, J. V., Controlling palladium morphology in electrodeposition from nanoparticles to dendrites via the use of mixed solvents. Nanoscale 2020, 12(42), 21757-21769.
38. Armstrong, E.; O'Sullivan, M.; O'Connell, J.; Holmes, J. D.; O'Dwyer, C., 3D Vanadium Oxide Inverse Opal Growth by Electrodeposition. Journal of The Electrochemical Society 2015, 162 (14), D605-D612.
39. Armstrong, E.; McNulty, D.; Geaney, H.; O'Dwyer, C., Electrodeposited Structurally Stable V2O5 Inverse Opal Networks as High Performance Thin Film Lithium Batteries. ACS Appl Mater Interfaces 2015, 7 (48), 27006-15.
40. Potiron, E.; Le Gal La Salle, A.; Verbaere, A.; Piffard, Y.; Guyomard, D., Electrochemically synthesized vanadium oxides as lithium insertion hosts. Electrochimica Acta 1999, 45 (1-2), 197-214.
41. Potiron, E.; Le Gal La Salle, A.; Sarciaux, S.; Piffard, Y.; Guyomard, D., e-V2O5: Relationships between synthesis conditions, material characteristics and lithium intercalation behavior. Journal of Power Sources 1999, 81-82, 666-669.
42. Li, J. M.; Chang, K. H.; Hu, C. C., The key factor determining the anodic deposition of vanadium oxides. Electrochimica Acta 2010, 55 (28), 8600-8605.