Abstract Text
Li-rich layered metal oxides, such as Li1.2Ni0.13Mn0.54Co0.13O2 offer much larger specific capacities ( >250 mAh g-1 ) in Li-ion batteries than conventional oxides (<200 mAh g-1) because of the redox of lattice O2- ions in addition to the cations. Observing the oxygen distortion is a key part to the understanding of oxygen redox. Direct imaging of the oxygen deformation using atomic resolution electron microscopy is challenging because of the low contrast from oxygen and beam damage. Electron ptychography is a phase-reconstruction method in 4D scanning transmission electron microscopy (STEM), forming high-quality phase images because of its high signal-to-noise ratio and dose efficiency, and ability to detect and correct residual aberrations in post-acquisition data processing. The ptychographic phase image is sensitive to both the heavy transition metal (TM) and the light O and Li atoms. Focused-probe electron ptychography allows simultaneous collection of aberration-corrected ADF image when recording the 4D datasets for phase retrieval. Both the ADF and ptychographic phase images enable direct interpretation of the phase image and identification of the oxygen atoms.
Introduction
In the oxygen oxidation of Li1.2Ni0.13Mn0.54Co0.13O2, the lattice O2- sublattice distorts in the bulk and surface region, with oxygen dimerization mainly occurring in the bulk and oxygen loss predominantly at the surface [1,2]. Direct imaging of the oxygen shift is challenging using atomic-resolution electron microscopy, resulting from the low-sensitivity to oxygen and beam damage. In transmission electron microscopy (TEM), the incident electrons into oxygen are weakly scattered and lead to small phase shift. High-angle annular dark field (HAADF) imaging in STEM shows contrast proportional to Z1.7 and so is insensitive to the light elements. Annular bright field (ABF) imaging can visualize both light and heavy elements over a wide range of specimen thickness using an annular detector in the bright-field (BF) region. However, ABF is not tolerant to residual aberrations and sample mistilt and requires time to tune the sample and microscope [3]. Exposing specimen under the beam leads to radiation damage. Coherent BF STEM imaging offers phase contrast image but restricted with sample thickness. Differential phase-contrast (DPC) imaging forms differential image where the atom columns are located at the inflection points between the highest and lowest intensity, rather than at the intensity maximum [4]. DPC requires integration of signals from a pair of segmented detectors, resulting in low contrast transfer and dose efficiency. Electron ptychography is a phase-reconstruction method in 4D STEM, forming high-quality phase images because of its high contrast-transfer function and dose efficiency [3]. Focused-probe electron ptychography allows simultaneous collection of aberration-corrected ADF image when recording the 4D datasets, enabling direct identification of oxygen. In this work, we show how to use focused-probe electron ptychography to investigate the average projected O-O distance of Li1.2Ni0.13Mn0.54Co0.13O2 at various stages in the first charge-discharge cycle and reveal the oxygen deformation in the lattice structure. Data were collected using aberration-corrected JEOL ARM200F microscope mounted with PNCCD/4DCanvas pixelated detector. Ptychographic reconstruction was performed using Matlab codes adapted from Wigner Distribution Deconvolution (WDD).
Results and discussion
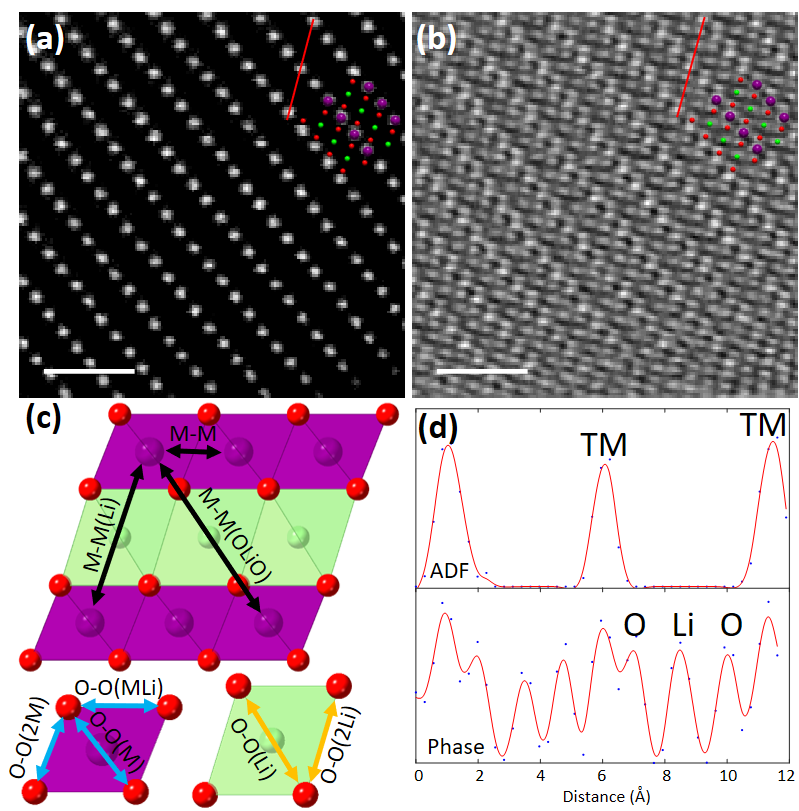
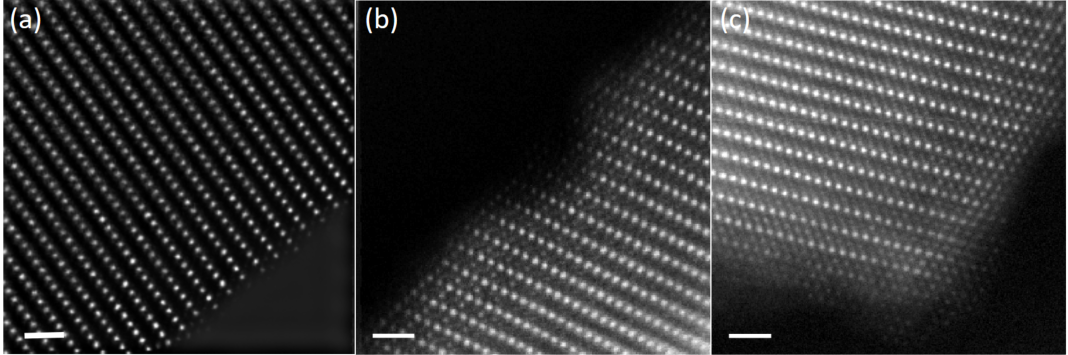
Figures 1a and b display the simultaneous HAADF and ptychographic phase image of pristine Li1.2Ni0.13Mn0.54Co0.13O2 projected parallel the layers. Between the TM layers, the contrast from O and Li atoms is invisible in the HAADF but revealed in the phase image, enabling direct measurement of the projected distance illustrated in Figure 1c. The statistical result of the atom distance is derived from multiple measurements using line profiles. Figure 1d shows the line profiles taken over the red lines. Such values from direct measurement can be distorted resulting from the sample drift during the slow-speed acquisition of the 4D datasets. To correct the values, we carried out multi-frame fast scanning of the HAADF images followed by rigid and non-rigid registration using SmartAlign [5] to generate zero-drift HAADF images and obtain the zero-drift TM-TM distances. Figures 2(a-c) display the zero-drift HAADF images of Li1.2Ni0.13Mn0.54Co0.13O2 at pristine, charged and discharged state, respectively. The zero-drift TM-TM distances can be used as a reference to calibrate the values directly measured from the ptychographic phase images of Li1.2Ni0.13Mn0.54Co0.13O2 at each charge-discharge stage. The measurement and calibration protocols generate zero-drift atom distances in Li1.2Ni0.13Mn0.54Co0.13O2. This approach allows measurement of the changes in the layer spacing and distortion of the O octahedra coordinating the TM species during the first charge-discharge cycle [6].
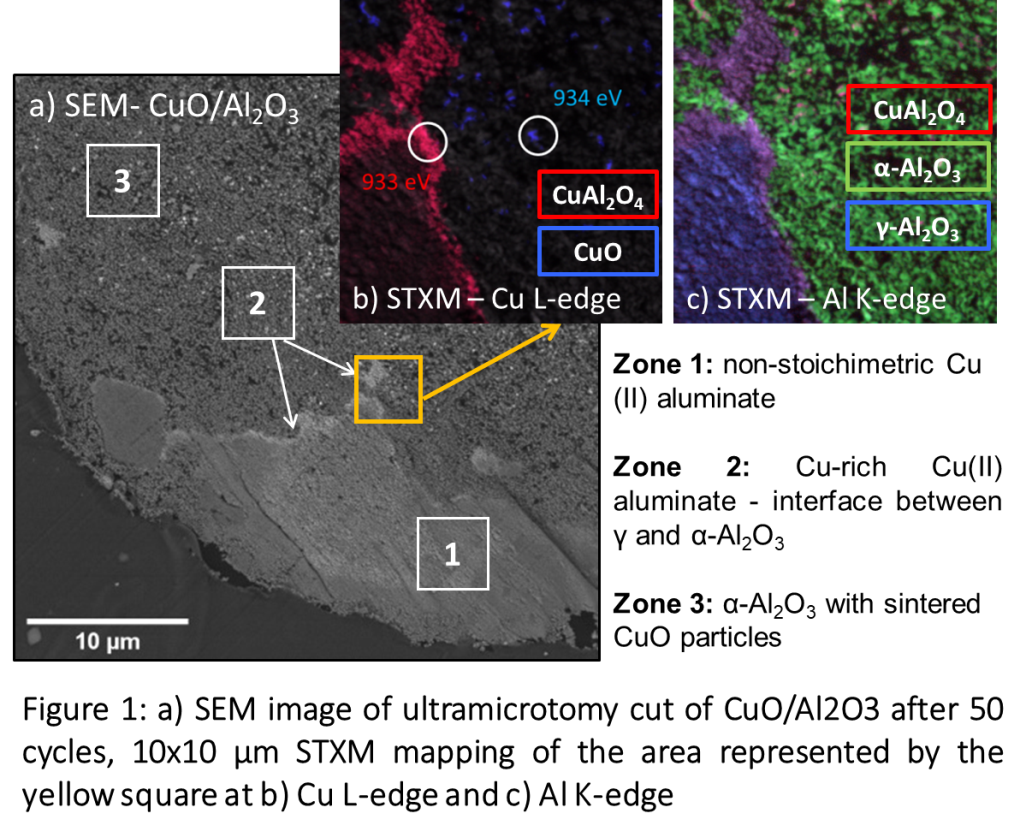
Figure 1 Measurement of projected atom distance in Li1.2[Ni0.13Mn0.54Co0.13]O2. Simultaneous (a) HAADF and (b) ptychographic phase image along [010] projection. Superposed is the crystal model, purple is TM, red O and green Li. Scale bar is 1 nm. (c) Illustration of atom distance. (d) Line profiles across the red lines in the HAADF and phase image.
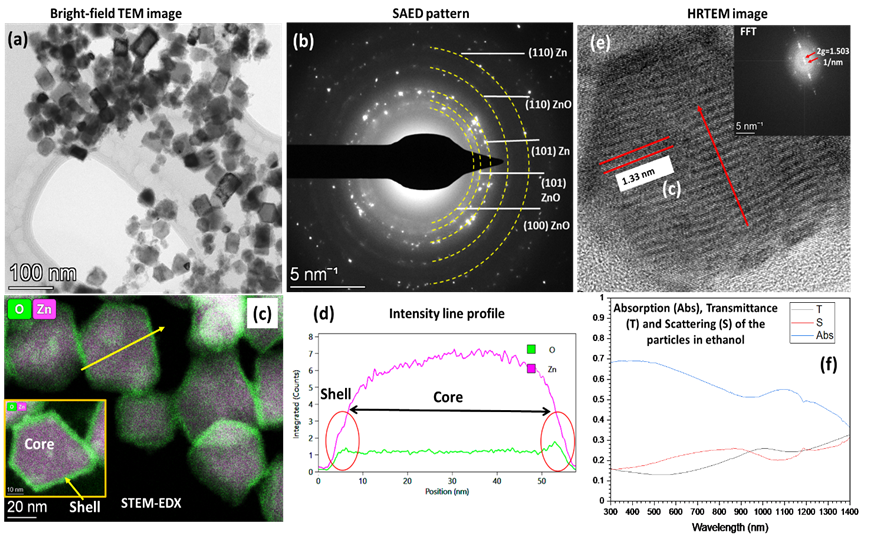
Figure 2 Atom distance measurement from zero-drift HAADF image of Li1.2[Ni0.13Mn0.54Co0.13]O2 at (a) pristine sate, (b) charged state and (c) discharged state. The dose is 3720, 2050 and 1943 e-/Å2, respectively. Scale bar is 1 nm.
Keywords
oxygen deformation; Li-ion battery; cathode; electron ptychography
References
[1] House, R. A. et al., Nature Energy 2020, 5 (10), 777-785.
[2] Liberti, E., et al., Ultramicroscopy 2019, 210, 112914.
[3] Lozano, J. G., et al, Nano Lett 2018, 18 (11), 6850-6855.
[4] Yang, H., et al, Ultramicroscopy 2017, 180, 173-179.
[5] Jones, L., et al, Advanced Structural and Chemical Imaging 2015, 1 (1), 8.
[6] The authors acknowledge use of characterization facilities within the David Cockayne Centre for Electron Microscopy, Department of Materials, University of Oxford and in particular the Faraday Institution (FIRG007, FIRG008), the EPSRC (EP/K040375/1 “South of England Analytical Electron Microscope”) and additional instrument provision from the Henry Royce Institute (Grant reference EP/R010145/1).