Abstract
Purpose: The utility of blue light cystoscopy (BLC) in patients receiving Bacillus Calmette Guerin (BCG) during post-treatment cystoscopy is not well understood. Our objective was to determine if BLC improves recurrence detection in non-muscle invasive bladder cancer (NMIBC) patients undergoing BCG.
Materials and Methods: Using the prospective multi-institutional Cysview registry (2014-2019), NMIBC patients who received BCG within 1 year prior to BLC were identified. Primary outcomes were recurrences and whether lesions were detected on white light cystoscopy (WLC), BLC, or both. To demonstrate the utility of BLC, we calculated the percentage of cystoscopies with recurrence that would have been missed with WLC alone. The false positive rate was the proportion of cystoscopies with biopsies only due to BLC suspicious lesions with benign pathology.
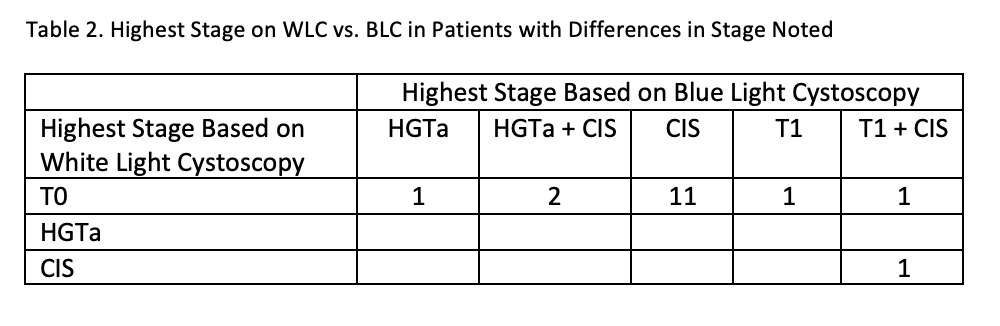
Results: From 1703 BLC procedures, there were 283 cystoscopies in the analytic cohort. The overall recurrence rate was 44.9% (n=127). If only WLC had been used, 13% (n=16) of recurrences would have been missed because 9% (n=16) of cystoscopies with normal WLC had recurrences identified with BLC. Among the 16 patients with recurrence missed with WLC, 88% (n=14) had CIS. The false positive rate was 4% (n=11).
Conclusions: BLC helped identify patients with recurrences after recent BCG that would have been missed with WLC alone. Providers should consider using BLC for high-risk patients undergoing BCG. As clinical trials for novel therapies for BCG unresponsive disease increase and there are no clear guidelines on BLC use for post-BCG cystoscopies, it is important to consider how variable BLC use could affect comparisons of these studies.