Abstract
Objectives: Catheterizable channels are used as a surgical treatment for pediatric patients with neurogenic bladder or congenital malformation of the urinary tract. There are several approaches for creation of catheterizable channels in pediatric urology using the Mitrofanoff principle. The appendix is most commonly utilized, however, the Monti ileovesicostomy can be performed when the appendix is unavailable for use. In this video we cover the important points in construction of the various channels.
Materials and Methods: The key steps of variations in approach for appendicovesicostomy and ileovesicostomy were recorded in pediatric patients. Procedure types include appendicovesicostomy with extravesical and split appendix approaches as well as ileovesicostomy with traditional Monti and spiral Monti approaches.
Results: The presented surgical techniques allow for durable results in pediatric patients and the variations in approach address the anatomical needs of each patient.
Conclusions: Creation of catheterizable channels via the presented techniques allow for intermittent catheterization via continent catheterizable stoma for pediatric patients.
Source of Funding: None
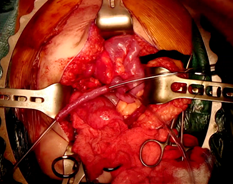
Figure 1 - Preparation of appendicovesicostomy channel.