Abstract
Objectives: Little is known about the prevalence of fistulas in patients with exposure to pelvic radiotherapy. The purpose of this systematic review and meta-analysis is to aggregate and summarize existing cohort study data on fistula prevalence among patients with a history of pelvic radiotherapy for pelvic malignancy.
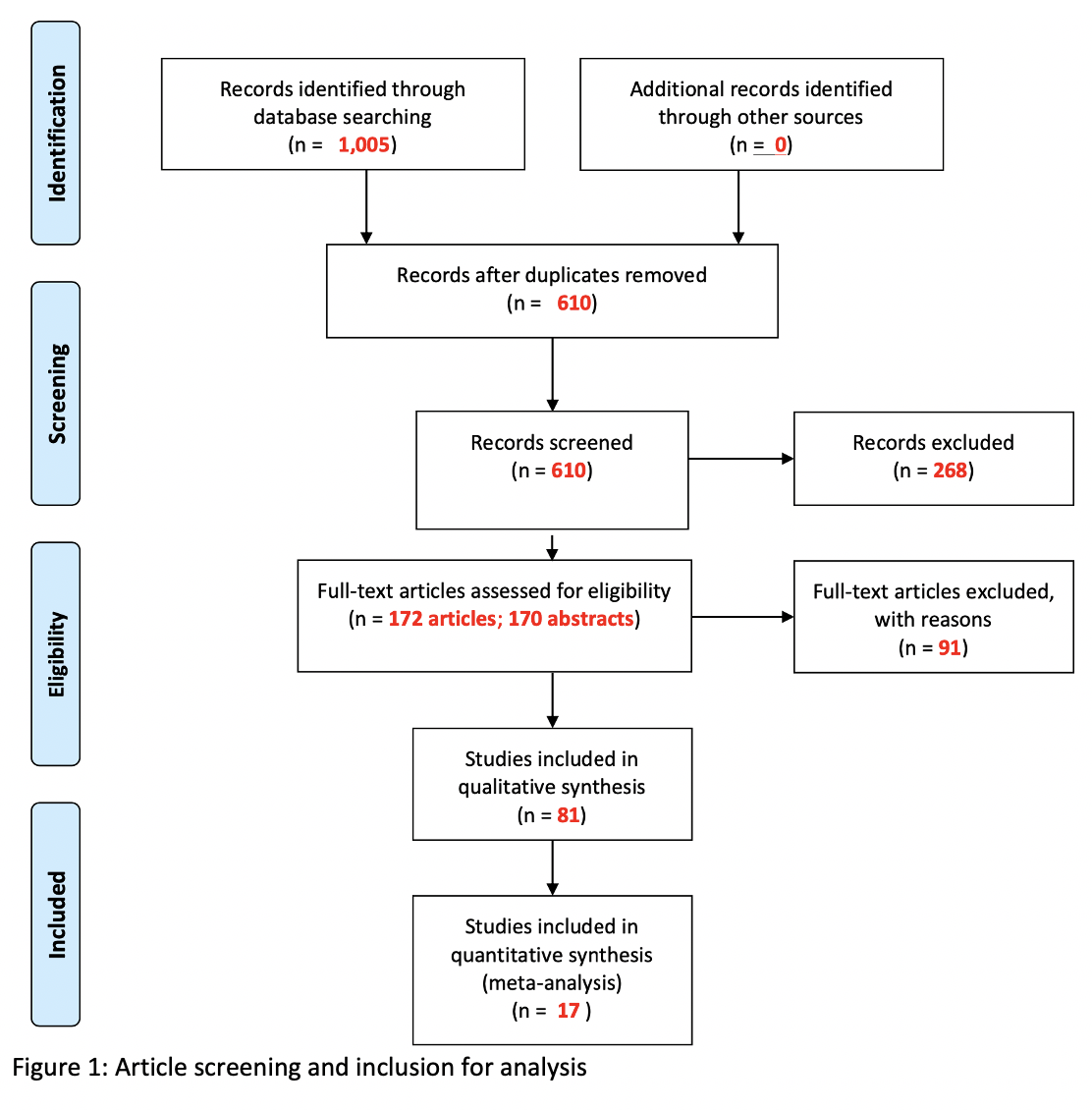
Materials and Methods: We queried PubMed, Embase, and Web of Science for studies pertaining to radiation induced fistulas in the pelvis. For this abstract, we included cohort studies reporting fistula prevalence among patients exposed to pelvic radiotherapy for the treatment of pelvic malignancy. We conducted meta-analysis using the fixed-effects model. We used I2 statistic to assess heterogeneity and the Newcastle-Ottawa Scale to assess risk of bias. PRISMA guidelines were followed, and our protocol was registered a priori on PROSPERO (CRD42020214451).
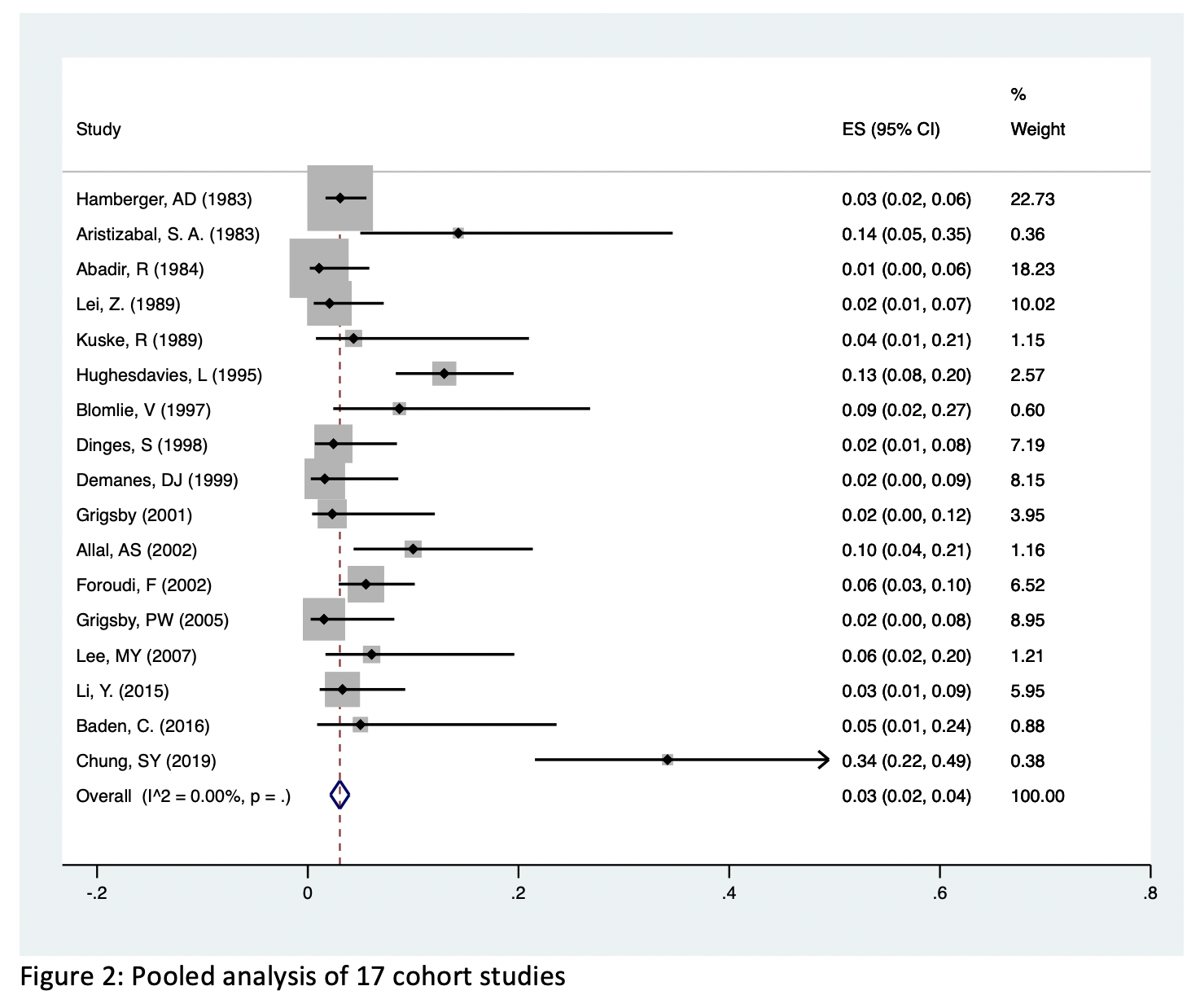
Results: We included 17 cohort studies with a total of 1,371 patients exposed to pelvic radiotherapy between 1983 and 2019. Mean patient age was 57.7 years and mean follow-up time was 32.5 months. The studies reported data on patients with cancer of the cervix (n=852), prostate (n=266), rectum (n=91), and multiple cancers (n=162). Pooled prevalence of radiation induced fistula was 3% (95% CI: 2% - 4%, I2 = 0%) and ranged between 1% and 34% in the included studies. Stratifying the pooled risk by year, cancer type, and fistula type demonstrated no significant heterogeneity between these substrata.
Conclusions: Patients undergoing pelvic radiotherapy for pelvic malignancy are at low risk for developing fistulas. The prevalence of fistulas has not changed over time and does not vary based on cancer type. Given that our mean follow-up time was 32.5 months, future studies should provide data on more long-term risk.