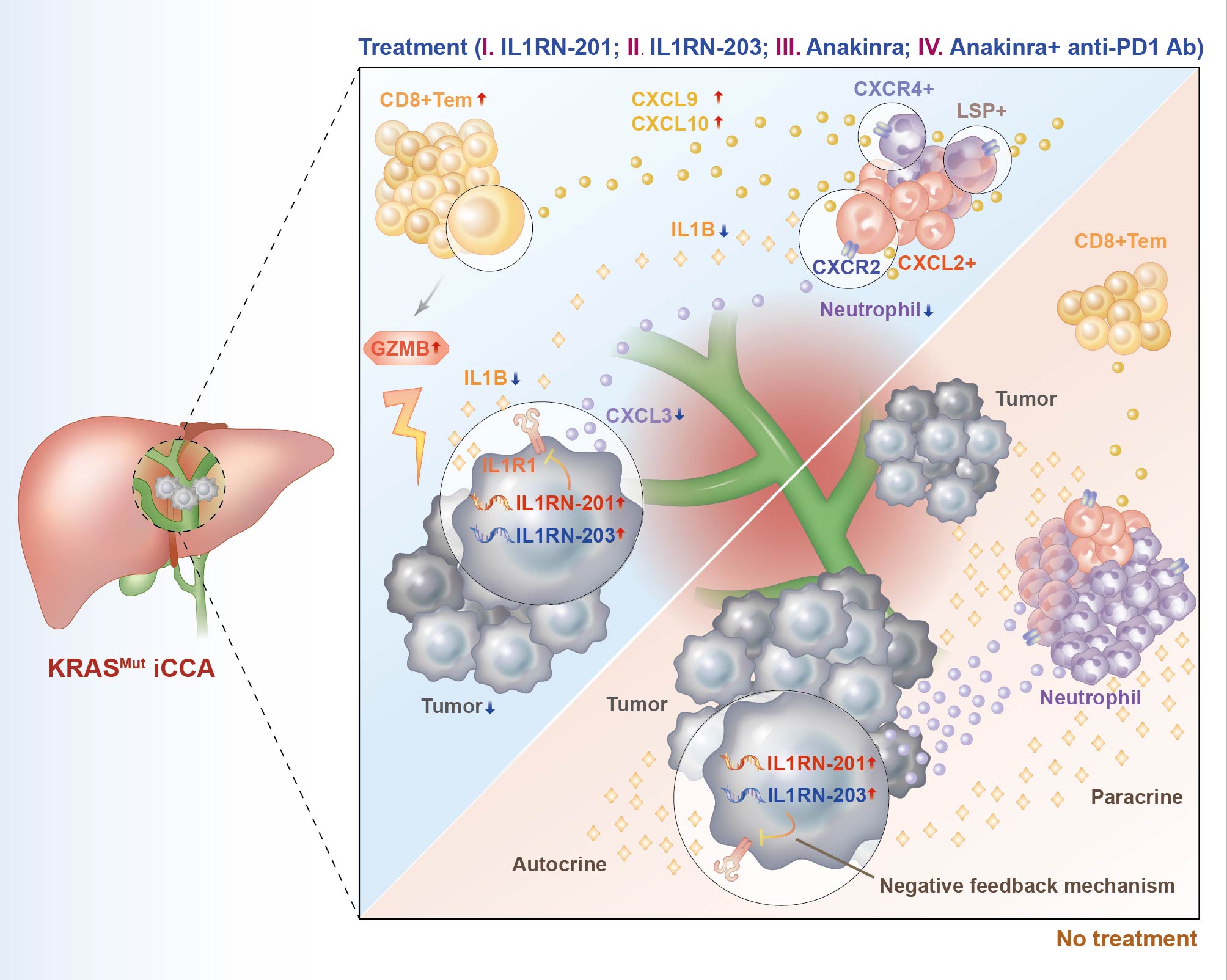
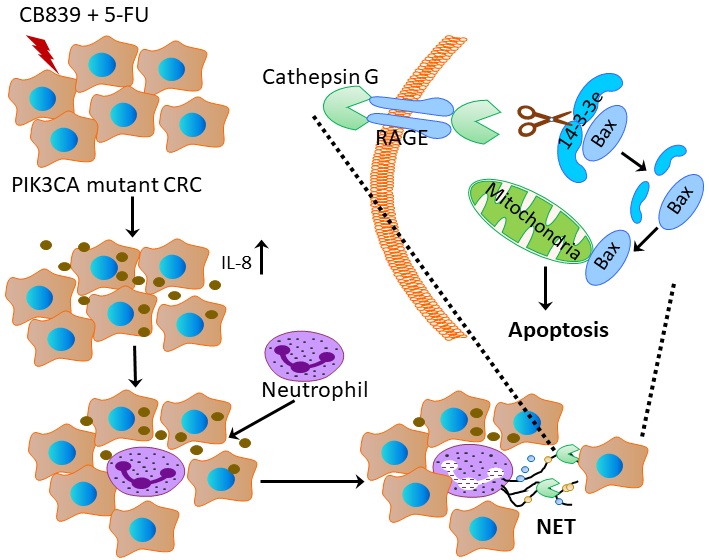
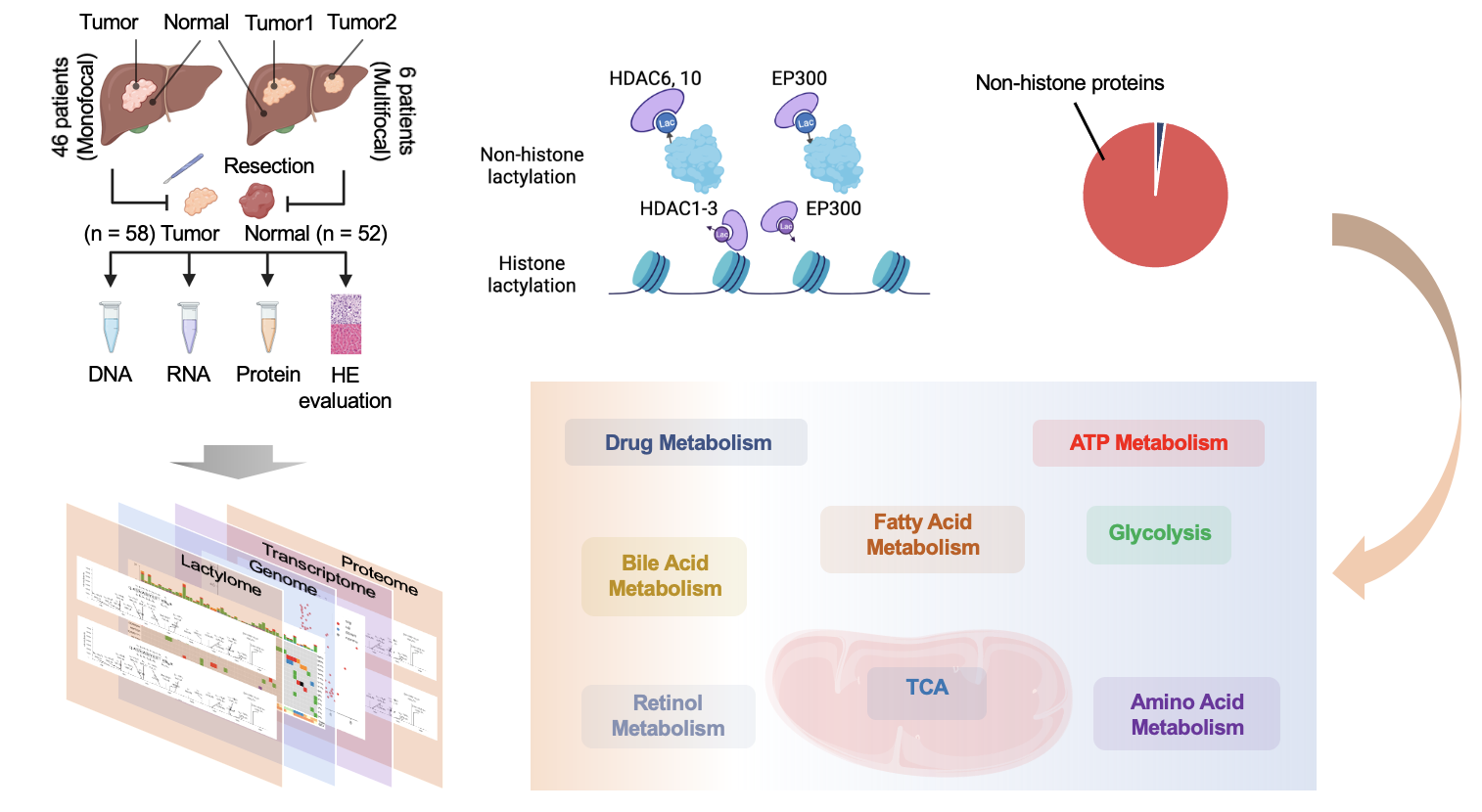
Activation of PI3K/AKT/mTOR signalling, most commonly by activating mutations of PI3K/AKT family members or loss of PTEN phosphatase function, contributes to carcinogenesis of many malignancies.
BTK (Bruton’s tyrosine kinase) is a non-receptor tyrosine kinase of the Tec kinase family, which is critical for B-cell development, differentiation and malignancies. Resistance frequently follows BTKi treatment. Reduced levels of BTK and upregulated PI3K/AKT signalling are hallmarks of these ibrutinib-resistant cells. Upregulation of PI3K-β expression is demonstrated to drive resistance in ibrutinib-resistant cells1.
The tumour suppressor PTEN is the major negative regulator of the PI3K/AKT signal. Loss of PTEN function is common in a number of cancers, including prostate. Preclinical studies have indicated that the PI3Kβ isoform is the critical lipid kinase that drives primarily PI3K pathway activation, cell growth, and survival in PTEN-deficient tumor cells2-6.
In this work, we explore the potential of PI3K-β/δ inhibitor CVL237(KA2237) in overcoming BTK-resistant B-cell lymphoma and PTEN-deficient prostate. CVL237 significantly inhibits the tumour growth in the BTK-resistant and PTETN-deficient xenograft model, suggesting that PI3K-β and PI3K-δ are the key nodes underlying AKT/mTOR axis signalling and cell survival in ibrutinib-resistant DLBCL and PTEN-null prostate.
CVL237, an oral dual selective PI3K-β/δ inhibitor, can reverse the resistance and inhibit tumour growth in the BTK-resistant xenograft mouse model. The effect is enhanced when combined with a BTK inhibitor.
Meanwhile, CVL237 exhibits antitumor activity in the PTEN-deficient CDX model and shows a synergistic effect with PARPi.
Some studies1 have demonstrated that reduced levels of BTK and compensatory upregulated PI3K/AKT pathway are potentially responsible for the emergence of ibrutinib-related resistance in DLBCL cells. Targeting the PI3K/AKT/mTOR axis with a PI3K-β/δ selective dual inhibitor reduced both tumorigenic properties and survival-based PI3K/AKT/mTOR signalling of ibrutinib-resistant cells.
Therefore, target inhibition of PI3K-β/δ is a potential therapy for BTK-resistant lymphomas and PTEN-deficient solid tumours.