Abstract Text
Misfit-layered compounds (MLCs) are stacks of two different layered materials, which have a lattice mismatch at least in one direction [1]. MLCs based on chalcogenides are composed of a metal chalcogenide (MX) and a transition-metal chalcogenide (TX2) and tend to form tubular structures due to the misfit stress and the elimination of dangling bonds upon seaming. Applying the chemical vapour transport (CVT) technique, MLC-nanotubes (NTs) can be synthesized with a high yield [2]. MLC-NTs are promising for applications, e.g. in the field of thermoelectricity, due to the complementary properties of the two layered compounds [3]. An improvement of the synthesis process of MLC-NTs allows alloying the compounds forming quaternary structures starting from LaS-TaS2 [4]. This work focusses on the electron microscopy study of the family of quaternary (La,Y)S-TaS2 nanotubes [5].
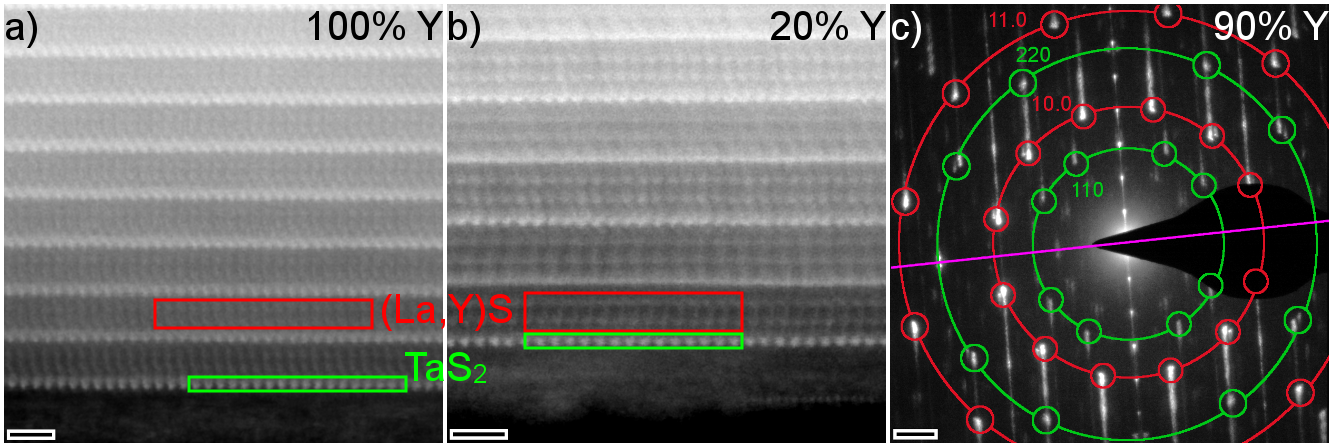
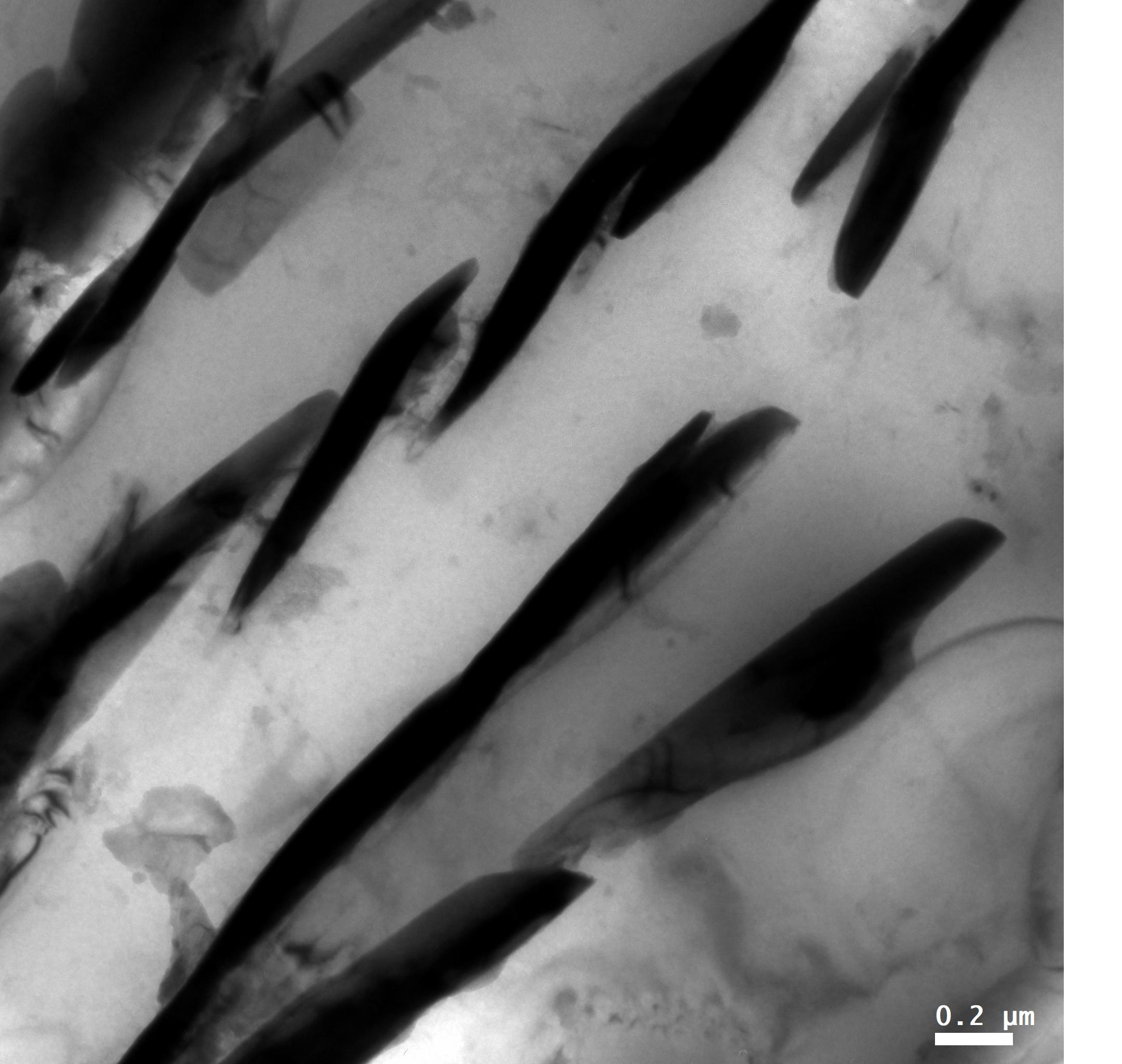
Using aberration-corrected transmission electron microscopy (TEM), we analysed the NTs by high-resolution (scanning) (S)TEM, electron diffraction (ED), energy-dispersive X-ray spectroscopy (EDX) and electron energy-loss spectroscopy (EELS) revealing their structure and composition. The synthesis of NTs was successful for the whole range of Y percentages between 0 to 100% with the NT yield being largest at 10% (global) and 80%. The two HAADF STEM images (Figure 1) reveal the layered misfit structure of one layer of TaS2 (TX2) and two layers of (La,Y)S (MX). While for pure NTs (0 and 100% Y) the layers were found to share the same orientation, quaternary structures often show two sets at different orientation that appear at double periodicity (alternating contrast of stacks in Figure 1b).
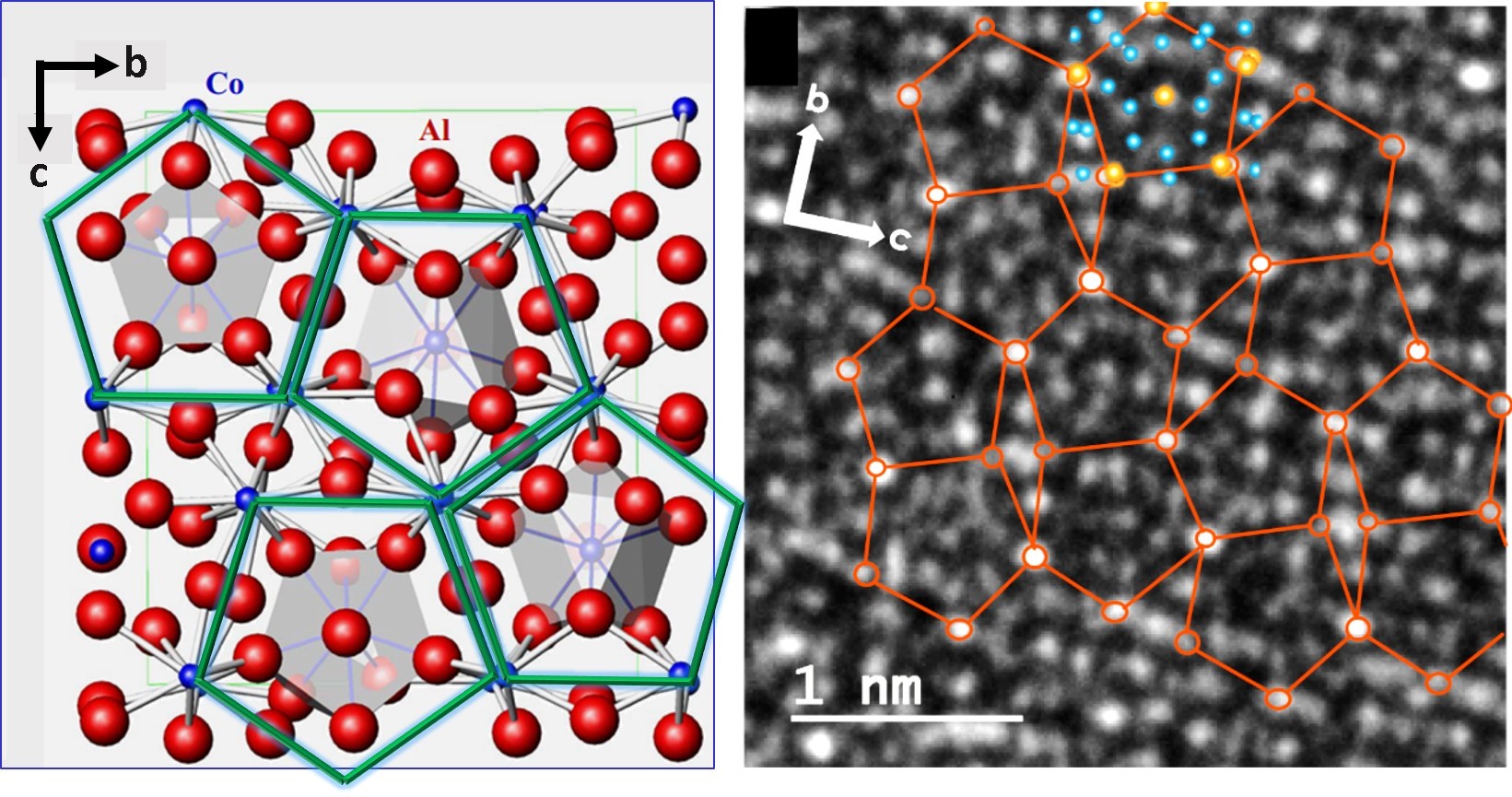
These predominant orientations are confirmed by ED results (Figure 1c), where 2x4 and 2x6 sets of reflections stemming from the (La,Y)S and TaS2 sublattices, rotated by 30º, are observed. ED also indicates a decreasing stack periodicity from 1.15 nm (0% Y) to 1.09 nm (100% Y) as well as a slightly shrinking lattice parameter of the (La,Y)S sublattice being in agreement when considering values of LaS and YS.
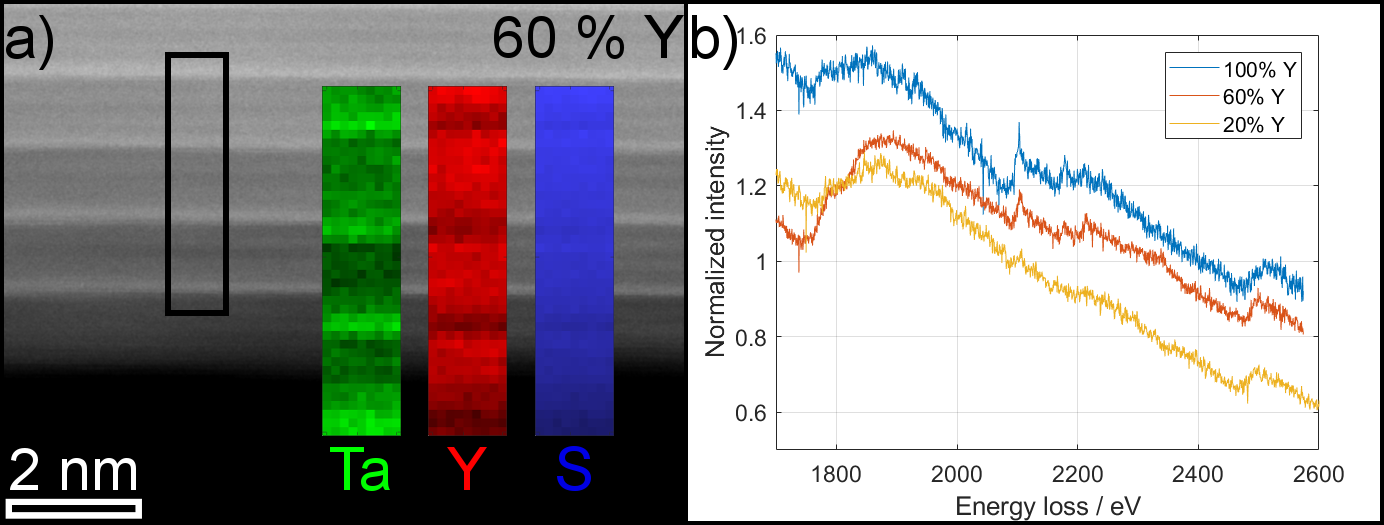
EELS measurements suggest a homogeneous and in-phase substitution of La by Y as shown by the spectrum imaging map in Figure 2a. The intensity of the Y-L edge at 2080 eV clearly increases with respect to the Ta edge for increasing Y content (Figure 2b). STEM-EDX results indicate a stoichiometric composition of the NTs for Y percentages >20% while only a reduced content of <5% Y is observed for an intended composition of La:Y 90:10 suggesting increased stability of Y-doped LaTaS NTs.
In conclusion, we were able to deduce the dependence of the structure and properties of the NTs on the Y content by in-depth EM analysis [5,6].
Figure 1: STEM images of different (La,Y)S-TaS2 NTs with (a) 100% Y showing a similar appearance of the layers and (b) 20% Y showing alternating contrast of the layers along c. (c) ED pattern of a 90% Y- NT showing 2x4 sets of 110 and 220 (La,Y)S reflections (green) and 2x6 sets of 10.0 and 11.0 reflections of TaS2 (red). Tube axis is indicated by the purple line and scale bars are (a,b) 1 nm and (c) 1 nm-1.
Figure 2: (a) EELS spectrum image for NT with 60% of Y revealing the spatial separation of Ta and Y in the MX and TX2 layers. (b) Comparison of EELS spectra normalized before Y-L edge shows increasing intensity of Y-L edge (2080 eV) with Y content as well as S-K (2472 eV) and Ta-M edge (1735 eV).
References
[1] GA Wiegers, Prog Solid State Ch 24 (1996), 1–139.
[2] G Radovsky et al.. Angew Chem Int Edit 50(2011), 12316–12320.
[3] M Serra, R Arenal, R Tenne, Nanoscale 11 (2019), 8073-8090.
[4] G Radovsky et al., J Mater Chem C 4 (2016), 89–98.
[5] S Hettler, M.B. Sreedhara, M. Serra, S. Sudarson Sekhar, R. Popovitz-Biro, I. Pinkas, A. N. Enyashin, R. Tenne and R. Arenal, submitted.
[6] Research supported by the Spanish MINECO (MAT2016-79776-P, AEI/FEDER, EU), Government of Aragon through project DGA E13_17R (FEDER, EU) and European Union H2020 program “ESTEEM3” (823717).