Abstract Text
Microbial cells produce a variety of storage materials, which serve as an energy source, carbon source, etc. Polyhydroxyalkanoates (PHA), polyesters of hydroxyalkanoic acids, are biodegradable plastics accumulated as granules in cells of many prokaryotic microorganisms. Primarily PHA are used in microbial cells as storage material, however, recent studies revealed, that cells containing PHA are also more resistant to stress environment such as osmotic imbalances, UV irradiance or temperature changes. Production of polyhydroxyalkanoates is induced in excess of carbon source and also limitation of another element essential for cellular growth. Besides microbial cell storage material, PHA are also considered as a promising bioplastic material, due to its biodegradability and mechanical properties similar to petrochemical plastics, such as polypropylene (PP). [1–3]
Currently, Cupriavidus necator or recombinant Escherichia coli are considered as one of the biggest PHA producers. Unfortunately, the production of PHA using heterotrophic microorganisms requires large amounts of carbon substrates, such as glucose or fructose, which makes the production quite expensive compared to PP. Carbon substrates could make up to 50% of all production costs. One of the possibilities, how to reduce these expenses, could be the use of waste material (eg. frying oils) as a carbon source or production of PHA by photoautotrophic microorganisms. [1,3,4]
Promising strain for PHA production is Synechosystis sp., unicellular cyanobacteria. Cyanobacteria are prokaryotic microorganisms commonly occurring in freshwater and also saltwater. Unlike heterotrophic bacteria Cupriavidus necator, Synechosystis salina contains pigments like phycocyanin, carotenoids and chlorophylls and performs photosynthesis. Therefore, these bacteria do not require organic carbon substrate and are able to convert CO2 into various valuable metabolites including PHA. However, compared to heterotrophic organisms, cyanobacteria are not able to produce that high amounts of PHA in their cells [3,4]. This study is focused on deeper morphological study of cyanobacterial cells of strains Synechocystis sp. PCC 6803 and Synechocystis salina CCALA 192, mainly on the content of intracellular PHA granules.
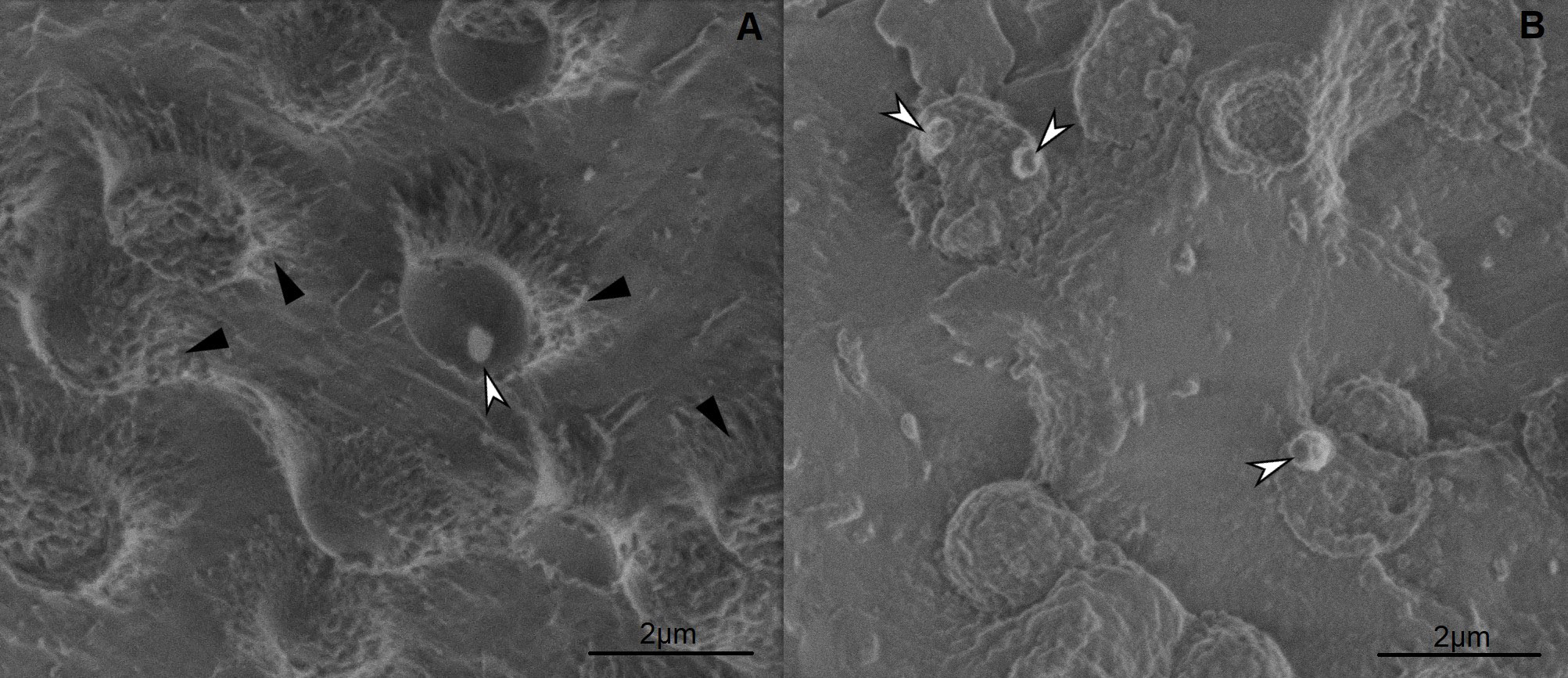
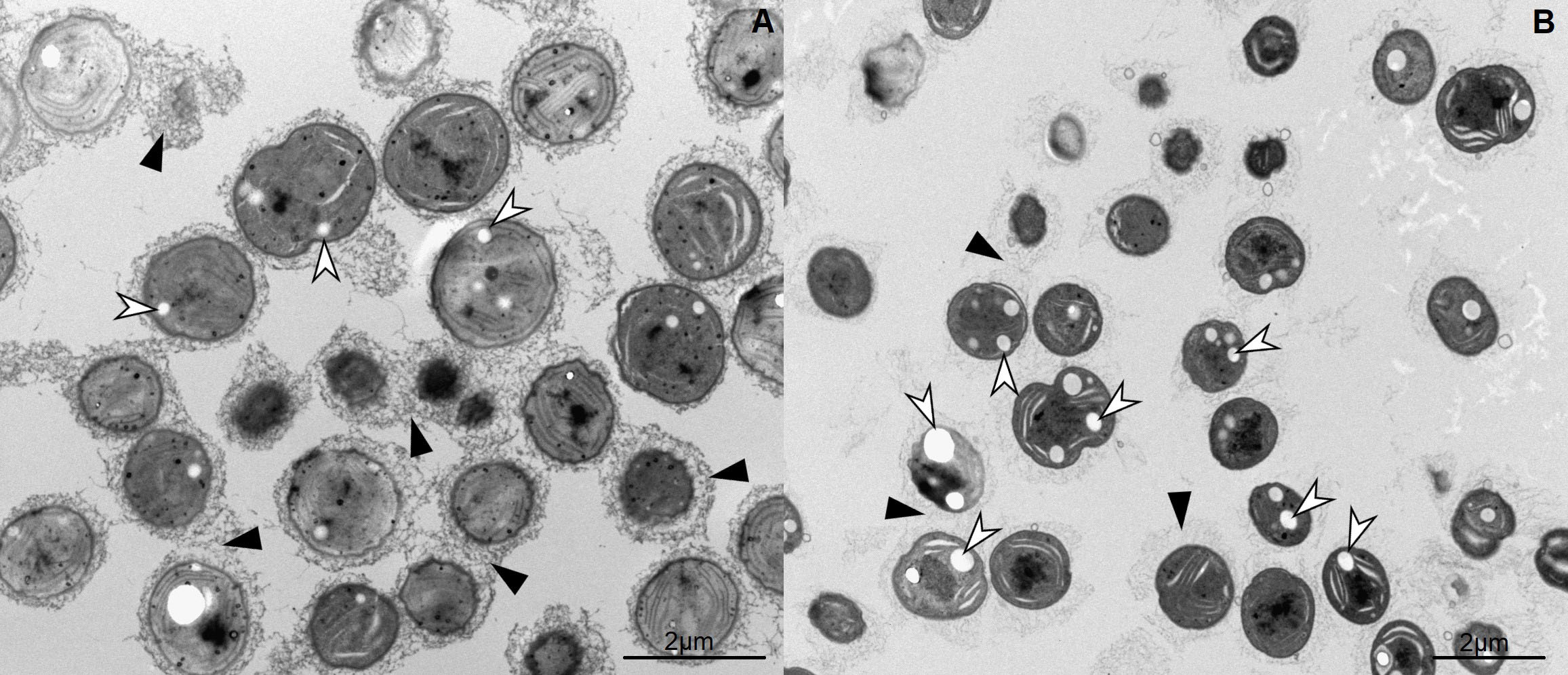
Cyanobacterial cells were fixed using high-pressure freezing method. After fixation samples were processed using either freeze-fracture method for observation in cryo-SEM or freeze substitution followed by embedding in epoxy-resin for observation in TEM. Freeze fracturing method enables the imaging of intracellular content of cells. PHA granules at temperatures of −130 °C remain elastic and can be observed in cryo-SEM sticking out of fractured cells. Comparison of Synechocystis sp. PCC 6803 and Synechocystis salina CCALA 192 (Figure 1) showed, that strain Synechocystis sp. PCC 6803 contains less PHA granules, but produces higher amount of extracellular substances, forming mass surrounding each cell. This observation was confirmed also by TEM images (Figure 2). After postfixation with OsO4, embedding in Epon and staining ultrathin sections with lead citrate and uranyl acetate images shows higher content of PHA granules in Synechocystis salina CCALA 192 strain, but extracellular matrix was observed mostly in Synechocystis sp. PCC 6803 samples. These findings prove, that cyanobacteria are perspective microorganisms for PHA production and should be further investigated.
Figure 1: cryo-SEM images of A) Synechocystis sp. PCC 6803, B) Synechocystis salina CCALA 192. Freeze fracturing followed with freeze etching revealed inner structure of the cyanobacterial cells, showing PHA granules (white arrows) and extracellular mass (black arrows).
Figure 2: TEM images of A) Synechocystis sp. PCC 6803, B) Synechocystis salina CCALA 192. Staining of ultrathin sections revealed white intracellular PHA granules (white arrows) and dark extracellular mass (black arrows), which is denser in Synechocystis sp. PCC 6803 strain.
References
[1] S. Obruca et al, Involvement of polyhydroxyalkanoates in stress resistance of microbial cells: Biotechnological consequences and applications. Biotechnology Advances 36 (2018) p. 856-870.
[2] P. Sedlacek et al, PHA granules help bacterial cells to preserve cell integrity when exposed to sudden osmotic imbalances. New Biotechnology 49 (2019) p. 129–136.
[3] C. Troschl et al, Cyanobacterial PHA Production—Review of Recent Advances and a Summary of Three Years’ Working Experience Running a Pilot Plant. Bioengineering 4 (2017) p. 26.
[4] C. Troschl et al, Pilot-scale production of poly-β-hydroxybutyrate with the cyanobacterium Synechocytis sp. CCALA192 in a non-sterile tubular photobioreactor. Algal Research 34 (2018) p. 116-125.
[5] The research was supported by the Czech Science Foundation (project GF19-29651L), the Technology Agency of the Czech Republic (project TN01000008); the infrastructure by the Czech Academy of Sciences (project RVO:68081731).