Abstract
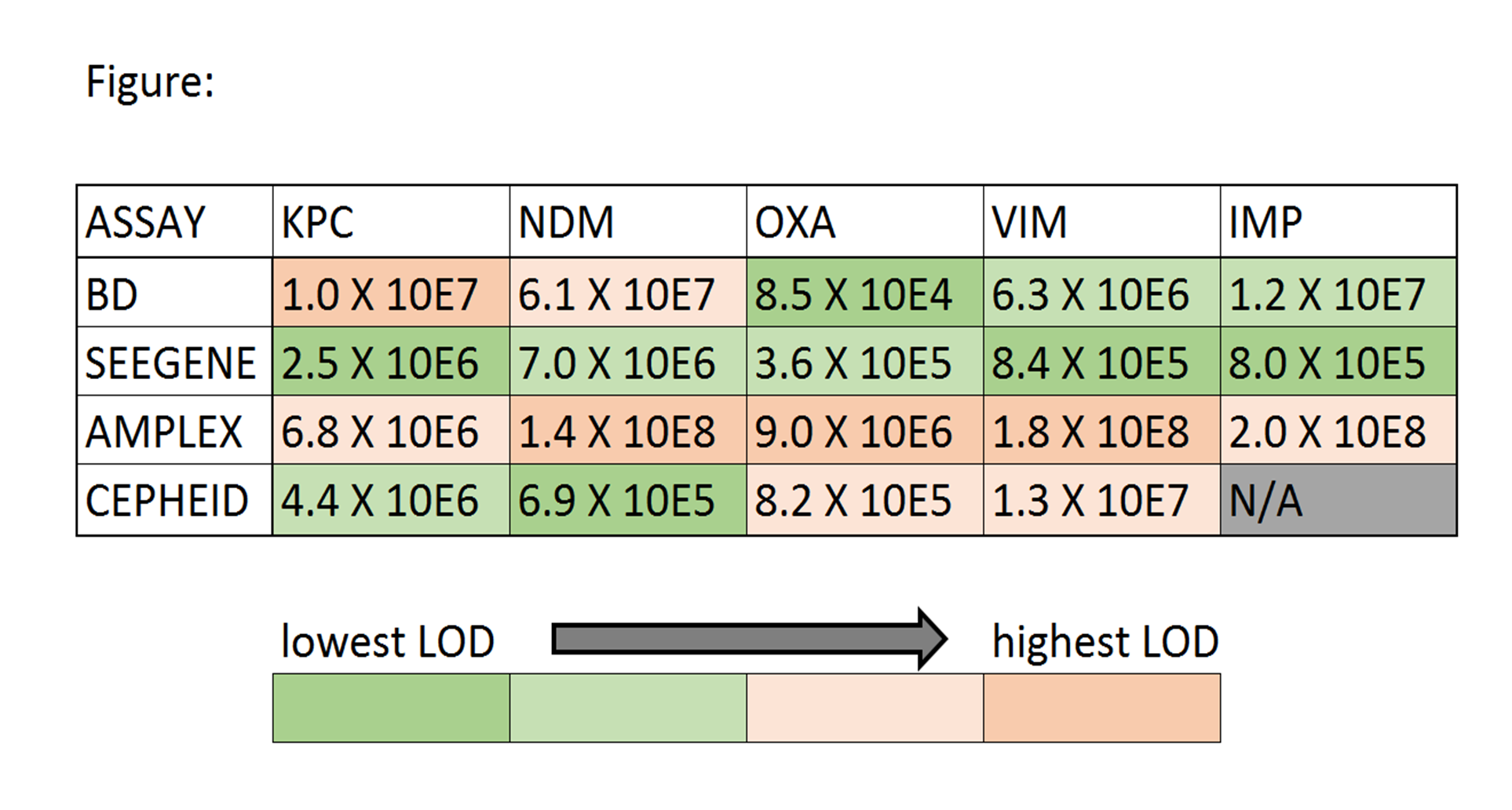
Objectives: Rapid detection of carbapenemase-producing organisms (CPO) is important. Data suggest direct-from-specimen NAAT is more sensitive than culture. We evaluated three multiplex carbapenemase NAAT targeting KPC/NDM/OXA48-like/ VIM/IMP genes [BDMax Checkpoints CPO (BD), Allplex Entero-DR (Seegene), and Easyplex® SuperBug CRE Assay Version C (Amplex Diagnostics)] using 150 well-characterized (phenotypic and PCR/sequencing) GNB. Methods: 150 GNB including 122 CPO (116 targeted-CPO: 88 KPC/8 NDM/5 OXA/5 NDM+OXA/7 VIM/3 IMP; 6 non-targeted CPO: 1 GES/4 SME/1 NMC), and 28 non-CPO were tested comprising 145 Enterobacteriacaeae/5 non-Enterobactericeae GNB. Isolates were recovered from -80oC under ertapenem-selective pressure. Limit of detection (LOD) was calculated in triplicate using four QC strains following manufacturer direct-from-specimen-protocols using 10E4/10E5/10E6/10E7/10E8 cfu/L concentrations in ESwab transport medium with results from Xpert® Carba-R (Cepheid) as reference. Colony counts confirmed concentrations and average LOD was calculated. Accuracy was determined using ≥10-fold-higher concentrations than calculated LODs. Discrepancies were repeated. Results: LOD (cfu/L) are shown (Figure). The most sensitive to least sensitive assay was Seegene, BD, Cepheid, then Amplex. All 34 non-targeted-CPO/non-CPO were negative by all assays. 1 KPC/1 NDM/3 KPC were initially missed by BD/Seegene/Amplex, respectively but were positive on repeat testing of fresh subcultures suggesting initial lost plasmids. 2 IMP were reproducibly missed by BD and Amplex. Final CPO-detection sensitivities/specificities were 100% for all non-IMP targets; respective 95%CI were: KPC 95.0-100/87.6-100; NDM 73.4-100/95.9-100; OXA48-like 68.0-100/96.0-100; VIM 59.6-100/96.1-100. Sensitivities/specificities for IMP were 100%(38.2-100)/100%(96.2-100)(Seegene) and 33%(5.6-79.8)/100%(96.2-100)(BD&Amplex).
Conclusions: All three assays were highly-accurate (100% sensitive/specific) for detection for KPC, NDM, OXA48-like and VIM CPO. IMP was more challenging for BD and Amplex. LOD was variable but not substantially different between assays. These results along with workflow, turn-around-time, footprint, interfaceability, cost, and laboratory needs can be used to determine suitability for different laboratories.
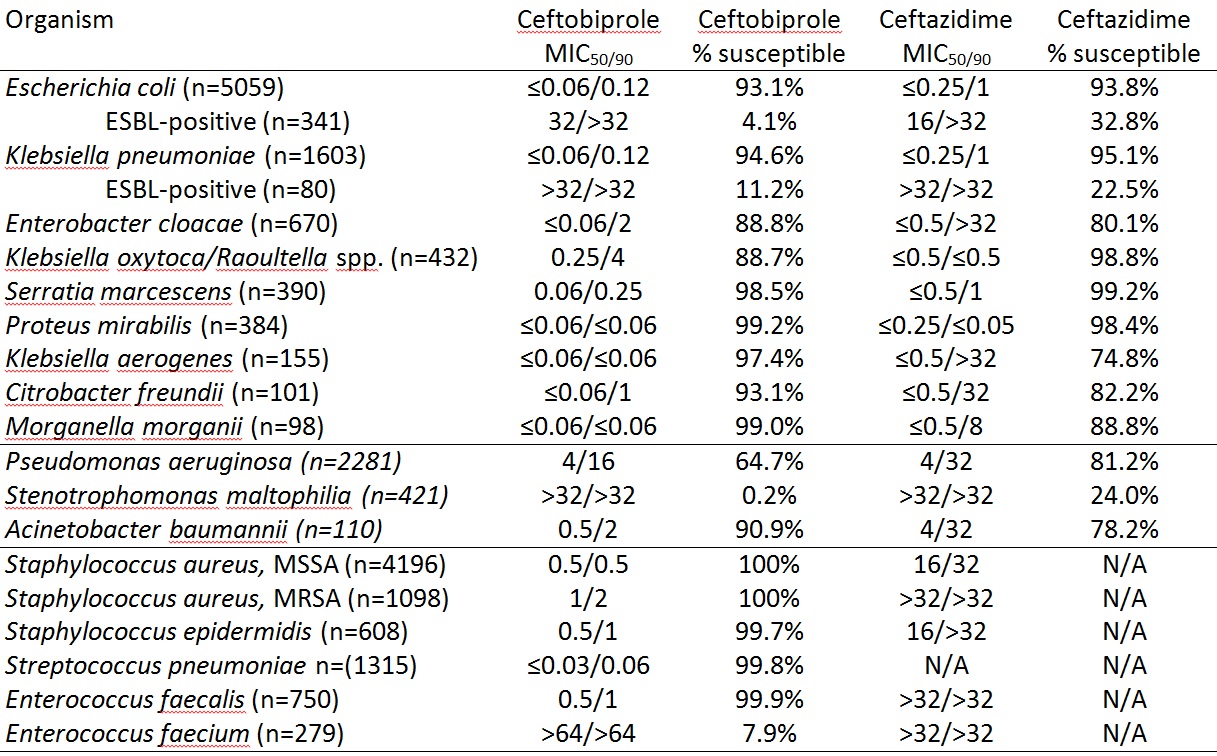 Conclusion: Ceftobiprole is active in vitro against S. aureus (MRSA and MSSA) and Enterobacteriaceae. It is more active in vitro than ceftazidime against Acinetobacter baumannii and species with chromosomal AmpC beta-lactamases while it is less active against Klebsiella oxytoca/Raoultella sp., suggestive that its chromosomal β-lactamase (OXY) is more active against ceftopiprole than ceftazidime. While ceftobiprole appears to have anti-Pseudomonas activity, no Pseudomonas-specific breakpoints exist and PK/PD breakpoints were derived using only one dosing recommendation (500mg IV q8h). Further studies in ceftobiprole dose, interval or infusion time may reveal that a higher species-specific breakpoint for Pseudomonas could be considered, concurrent with alternative dosing recommendations. Activity is poor against Enterococcus faecium and Stenotrophomonas maltophilia.
Conclusion: Ceftobiprole is active in vitro against S. aureus (MRSA and MSSA) and Enterobacteriaceae. It is more active in vitro than ceftazidime against Acinetobacter baumannii and species with chromosomal AmpC beta-lactamases while it is less active against Klebsiella oxytoca/Raoultella sp., suggestive that its chromosomal β-lactamase (OXY) is more active against ceftopiprole than ceftazidime. While ceftobiprole appears to have anti-Pseudomonas activity, no Pseudomonas-specific breakpoints exist and PK/PD breakpoints were derived using only one dosing recommendation (500mg IV q8h). Further studies in ceftobiprole dose, interval or infusion time may reveal that a higher species-specific breakpoint for Pseudomonas could be considered, concurrent with alternative dosing recommendations. Activity is poor against Enterococcus faecium and Stenotrophomonas maltophilia.