Photo upload

Abstract
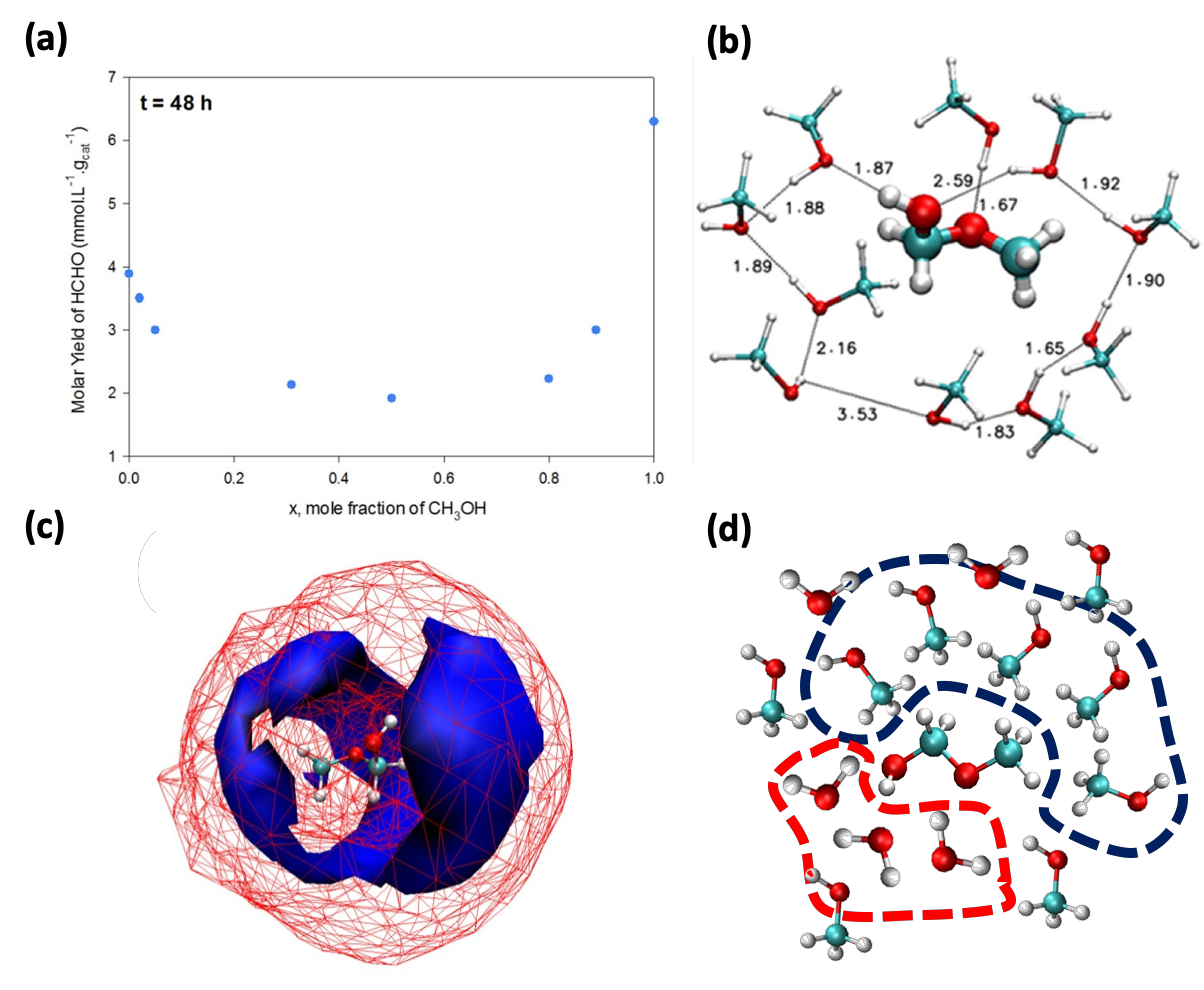
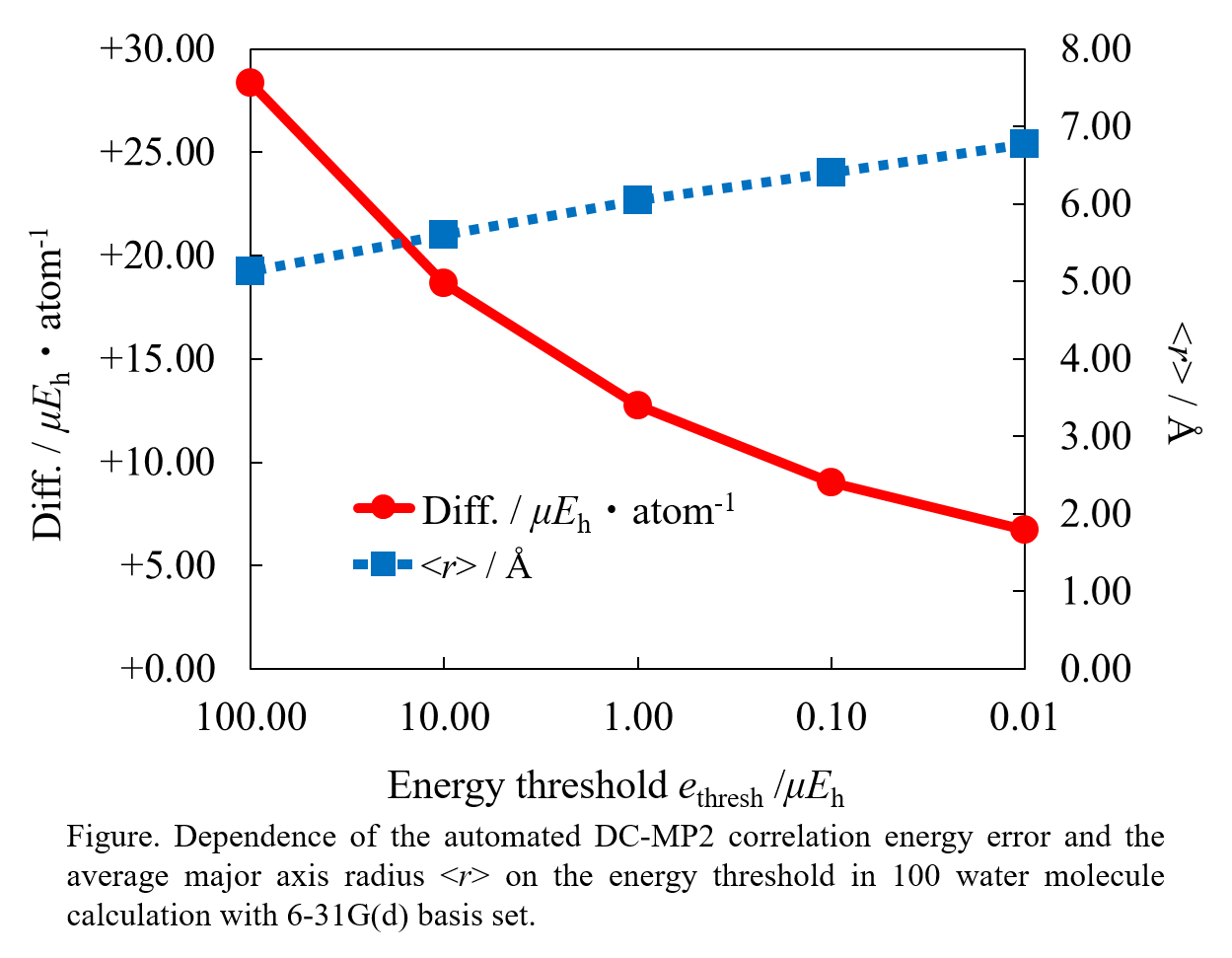
Anthropogenic carbon dioxide (CO2) emissions are a major contributor to rising global temperatures. The adsorption of CO2 inside Metal-organic frameworks (MOFs), a type of porous material, is a promising way to reduce atmospheric CO2 levels. Here, we study two MOFs; LMOF-201 and LMOF-202. Previous studies on both Zn-LMOF-202 and Zn-LMOF-201 reveal that both are isostructural, but Zn-LMOF-202 is rigid, whereas Zn-LMOF-201 is flexible. The change in MOFs’ properties by manipulation of their metal cations, organic linkers, and functional groups maintaining the structure topology, makes them highly suitable for CO2 adsorption. In this work, we propose a novel methodology to enhance the adsorption of CO2 in MOFs by post-synthetic metal exchange employing Grand Canonical Monte Carlo (GCMC) simulations. Using this computational method, we show that the CO2 adsorption capacity and selectivity can be magnified by a factor of about 1.5 by changing the metal ion from Zn to In in M-LMOF-202 at 1 bar and 298K. Furthermore, in past, it was found that slight modifications in the ligand part of the LMOF-202 framework by incorporating one oxygen atom into the structure, resulted in flexible LMOF-201. Due to this flexibility, the CO2 adsorption amount doubled at saturation compared to that of LMOF-202 at 195K. We introduce an approach combining Reactive force field (ReaxFF) and Molecular Dynamics (MD) simulations to capture the structural flexibility of LMOF-201 in presence of CO2 adsorption. We reproduce the experimental adsorption isotherms of CO2 in LMOF-201 and observe the existence of two different structures at low and high CO2 loading respectively. The results of this work show that both LMOF-201 and LMOF-202 have the requisite properties to merit further consideration as a carbon capture adsorbent